- Title
-
The Thermal Stability of the Collagen Triple Helix Is Tuned According to the Environmental Temperature
- Authors
- Fujii, K.K., Taga, Y., Takagi, Y.K., Masuda, R., Hattori, S., Koide, T.
- Source
- Full text @ Int. J. Mol. Sci.
|
Analysis of extracellular collagen and intracellular HSP47 proteins in ZF4 cells cultured at different temperatures. (a) The loaded amount of SDS samples prepared from culture media was normalized to the protein amount of the cell layer. SDS samples are loaded onto SDS-PAGE gel (5%) under reducing conditions. After transfer to a nitrocellulose membrane, collagen polypeptides were detected by using biotinylated soCMP6-7(Glu)2. (b) The culture media of ZF4 cells was treated with pepsin at 4 °C, and the triple-helical region of collagen was purified through salt precipitation. SDS-PAGE (5%) was performed under nonreducing conditions. Proteins were visualized with CBB. (c) HSP47 was pulled down from the lysate of ZF4 cells using type I collagen-coupled beads or mock-coupled beads. The SDS samples were subjected to SDS-PAGE (10%) under nonreducing conditions, and western blotting was performed using an antibody against HSP47 (top). The same membranes were reprobed with the anti-β-actin antibody (bottom). |
|
Quantification of total post-translational modifications in type I collagen secreted from ZF4 cells. Culture media of ZF4 cells grown at 4 °C was pepsin-treated, and collagen was purified through salt precipitation. Purified collagen was subjected to SDS-PAGE and transferred to a PVDF membrane. Then, α1(I) + α3(I) and α2(I) bands were excised and subjected to acid hydrolysis with SI-collagen. Pro, Lys, 3-Hyp, 4-Hyp, and total Hyl (Hyl + glycosylated Hyl) were quantified using LC-MS. Values for 4-Hyp (4-Hyp/(Pro + 4-Hyp + 3-Hyp)), total Hyl (total Hyl/(Lys + total Hyl)), and 3-Hyp (3-Hyp/(Pro + 4-Hyp + 3-Hyp)) ratios were calculated. Values are means ± SD (n = 3). Differences of means were assessed by one-way ANOVA followed by a Tukey’s post-hoc test. |
|
Quantification of site-specific 3-Hyp in type I collagen secreted from ZF4 cells. After pepsin treatment of culture media of ZF4 cells at 4 °C, collagen was purified through salt precipitation. Purified collagen was heat denatured and trypsin digested. LC-MS analysis was performed to quantify 3-Hyp at specific sites (indicated as boldface letters in the sequences). O indicates 4-Hyp. Values are means ± SD (n = 3). Differences of means in the 0 × 3-Hyp (unmodified Pro) were assessed by one-way ANOVA followed by Tukey’s post hoc test. |
|
Quantification of type I collagen α-chains secreted from ZF4 cells. Pepsinized collagen was purified from the culture media through salt precipitation. The samples were digested with trypsin after adding stable isotope-labeled synthetic peptides and heat denatured. The amount of each α-chain of type I collagen was determined through LC-MS analysis of marker peptides. Values are means ± SD (n = 3). Differences of means were assessed by one-way ANOVA followed by Tukey’s post hoc test. |
|
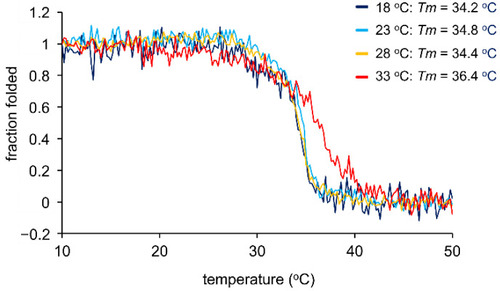
Thermal melting curves for collagen triple helix obtained by CD spectrometry. ZF4 cells were cultured for 3 days at the temperatures indicated. After the culture media were treated with pepsin at 4 °C, collagen was isolated by salt precipitation. CD signal was measured at 221 nm with increasing temperature (0.25 °C/min). The melting temperature (Tm) was set to the temperature at which the fraction folded was 0.5. |
|
A possible mechanism for tuning triple-helix stability according to the environmental temperature. When ZF4 cells were cultured below 28 °C, the thermal stability of the procollagen triple helix is almost constant because of the contribution of co-translational modifications. At a higher temperature of 33 °C, the additional mechanism to ensure thermal stability of procollagen triple helix is executed. |