- Title
-
Low Temperature Effect on the Endocrine and Circadian Systems of Adult Danio rerio
- Authors
- Sua-Cespedes, C.D., David, D.D., Souto-Neto, J.A., Lima, O.G., Moraes, M.N., de Assis, L.V.M., Castrucci, A.M.L.
- Source
- Full text @ Front. Physiol.
|
Experimental design. (A) Danio rerio male adults (n = 144) were divided in two large groups: a control temperature group (animals maintained at 28oC during 5 days and challenged 6 days at the same temperature) and a lower temperature group (animals maintained at 28oC during 5 days and challenged 6 days at lower temperature 23oC). After stimulation the animals were euthanized at early light (EL, 10 AM) and early dark (ED, 12 AM). (B) For the analysis, fish were separated into another two groups: a qPCR (96 animals) and a cortisol (48 animals) group. For the qPCR we evaluated gh1, aanat2, mtnr1aa, mtnr1bb, opn4m1, opn4m2 in the brain, ghra, ghrb, igf1a, igf1b, igf1ra, igf1rb, gr in the liver and muscle. Also, in all tissues analyzed we performed a qPCR for clock genes (per and cry). Each experimental n comprised pooled tissues from four fish. The total number of samples used in each temperature and time point was n = 3–6. |
|
Early light (EL) and early dark (ED) gene expression of growth hormone (gh1) and growth hormone receptors (ghra and ghrb) in adult Danio rerio. (A) Brain gh1; (B) liver ghra; (C) muscle ghra; (D) liver ghrb; (E) muscle ghrb. The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3–6 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
Early light (EL) and early dark (ED) gene expression of insulin-like factors (igf1a and igf1b), and their receptors (igf1ra and igf1rb) in adult Danio rerio. (A) Liver igf1a; (B) muscle igf1a; (C) liver igf1b; (D) muscle igf1b; (E) liver igf1ra; (F) muscle igf1ra; (G) liver igf1rb; (H) muscle igf1rb. The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3–6 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
Early light (EL) and early dark (ED) whole body cortisol and gene expression of glucocorticoid receptor (gr) in adult Danio rerio. (A) Cortisol levels; (B) liver gr; (C) muscle gr. The hormone concentration (μg) was normalized by sample weight (g, Supplementary Table S2). The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
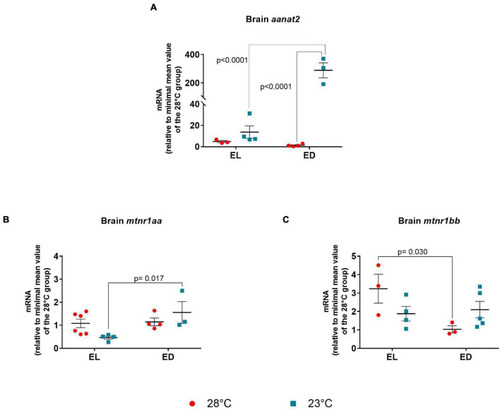
Early light (EL) and early dark (ED) gene expression of arylalkylamine N-acetyltransferase (aanat2) and melatonin receptors (mtnr1aa and mtnr1bb) in adult Danio rerio. (A) Brain aanat2; (B) Brain mtnr1aa; (C) brain mtnr1bb. The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3–6 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
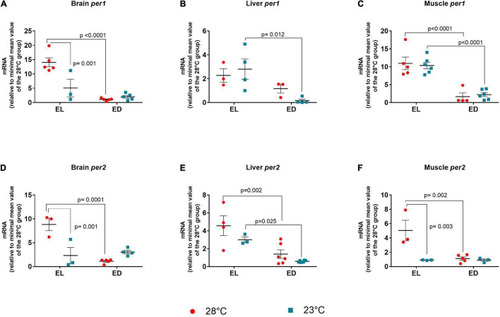
Early light (EL) and early dark (ED) expression of period (per1 and per2) genes in adult Danio rerio. (A) Brain per1; (B) liver per1; (C) muscle per1; (D) brain per2; (E) liver per2; (F) muscle per2. The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3–6 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
Early light (EL) and early dark (ED) expression of cryptochrome (cry1a and cry1b) genes in adult Danio rerio. (A) Brain cry1a; (B) liver cry1a; (C) muscle cry1a; (D) brain cry1b; (E) liver cry1b; (F) muscle cry1b. The gene expression was normalized by the 18S rRNA and expressed relative to the lowest mean of the control temperature group. The values are expressed as mean ± SEM. n = 3–6 (pools of four animals each). Significant differences are shown when p < 0.05. Red symbol corresponds to 28oC and blue symbol corresponds to 23oC. |
|
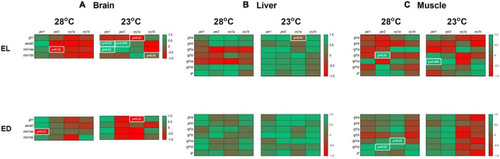
Correlation of gene expression in the brain, liver and muscle of adult Danio rerio. (A) Brain. Four positive and four negative correlations; (B) liver. One positive correlation; (C) muscle. Four positive correlations. Data from the same sample, temperature and time point with a Gaussian distribution were analyzed by Pearson correlation coefficients. The values are expressed as coefficient r. Values: 1 perfect correlation; 0 to 0.99 the two variables tend to increase or decrease together; 0 the two variables do not vary together at all; 0 to –0.99 one variable increases as the other decreases and –1 as perfect negative or inverse correlation. Significance was set for p < 0.05, shown in white letters inside white boxes. |
|
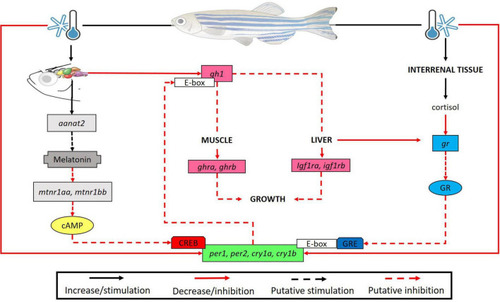
Brain of D. rerio submitted to low temperatures show high levels of the aanat2 transcripts causing a putative increase in melatonin synthesis. We suggested that expression of melatonin receptors (mtnr1aa and mtnr1bb) may decrease to ensure homeostasis. In turn, it is known that mtnr1aa, mtnr1bb (Gi protein-coupled receptors) inhibit adenylate cyclase activity which ultimately leads to the inhibition of CREB phosphorylation, a transcription factor required for clock gene expression, resulting in a reduction of their transcripts. Also, in the brain we found a reduction in the gh1 transcripts at lower temperature, leading to a decrease of ghra and ghrb in the muscle, and igf1ra, igf1rb in the liver, suggesting a long-term inhibition of the animal growth. In turn, clock genes are capable to bind to the promoter E-box region of genes as gh1 thus modulating its expression (a putative inhibition in our study). On the other hand, there was an increase in the cortisol concentration and a compensatory reduction of its receptor transcripts. The glucocorticoid (Gc)/glucocorticoid receptor complex modulates the expression of clock genes by binding to glucocorticoid response elements (GRE) in their promoters. Consequently, we evidenced a decrease in the expression of clock genes in all organs analyzed. This may be associated to cold-modulation by melatonin and cortisol pathways or a direct effect of low temperature on clock gene transcripts. |