- Title
-
Alpha-synuclein aggresomes inhibit ciliogenesis and multiple functions of the centrosome
- Authors
- Iqbal, A., Baldrighi, M., Murdoch, J.N., Fleming, A., Wilkinson, C.J.
- Source
- Full text @ Biol. Open
|
|
|
|
|
|
|
|
|
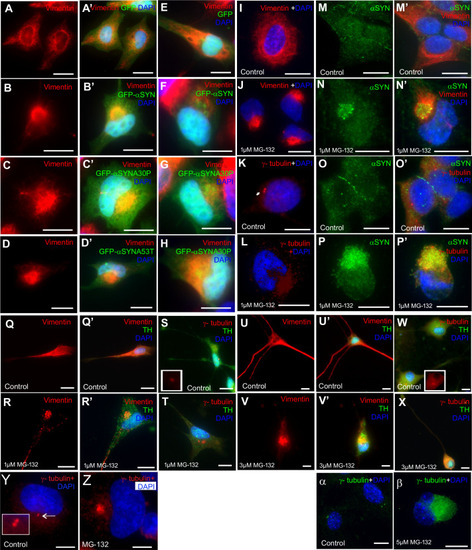
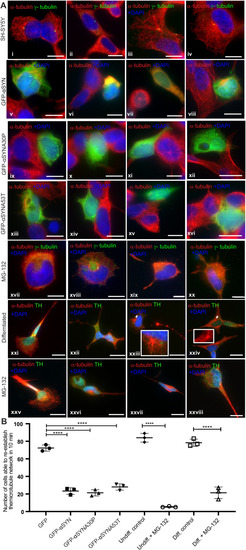
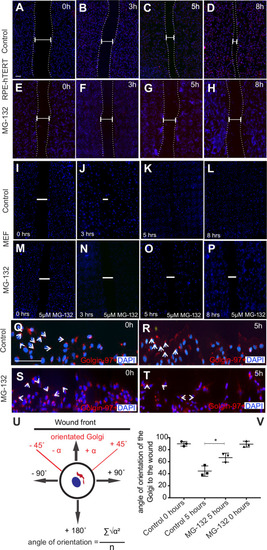
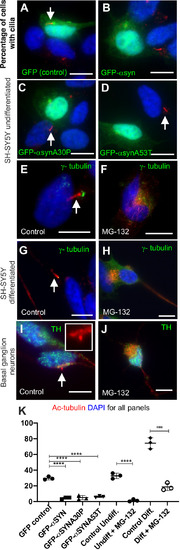
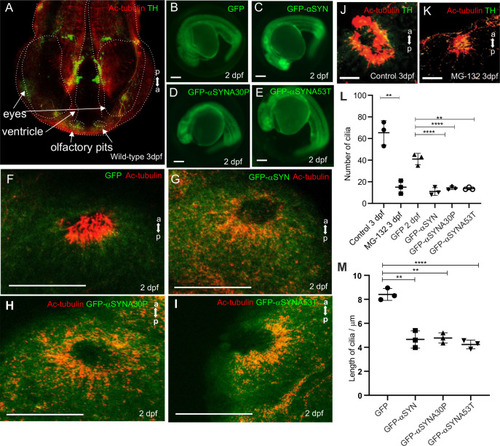
Olfactory cilia in zebrafish larvae are severely reduced in the presence of aggresomes. (A) The neuronal dopaminergic network in 3 dpf zebrafish forebrain viewed from the dorsal aspect, detected by TH-staining (green). Acetylated tubulin (red) stains axon tracts and cilia. (B–E) Overexpression of control GFP or α-syn, α-synA30P, α-synA53T familial mutations does not cause any anatomical defects in zebrafish larvae. (F) By 3 dpf extensive numbers of cilia are visible at the olfactory pit. (G–I) Overexpression of any of the three forms of α-syn (wild type, A30P or A53T) severely reduces numbers of cilia in the olfactory pit. Cilia length is also reduced. (J,K) Embryos treated with MG132 showed an extensive reduction in number of cilia. (L) Quantification of cilia numbers from above experiments (P=0.001, by one-way ANOVA, n=3). Cilia were counted from the confocal generated z-stacks, projected images of which are shown in this figure. (M) Quantification of length of cilia from F-I (P=0.0088, by one-way ANOVA, n=3). Scale bars: 100 μM. EXPRESSION / LABELING:
|