- Title
-
Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating β-catenin, leading to a decrease in endotoxic shock
- Authors
- Molagoda, I.M.N., Jayasingha, J.A.C.C., Choi, Y.H., Jayasooriya, R.G.P.T., Kang, C.H., Kim, G.Y.
- Source
- Full text @ Sci. Rep.
|
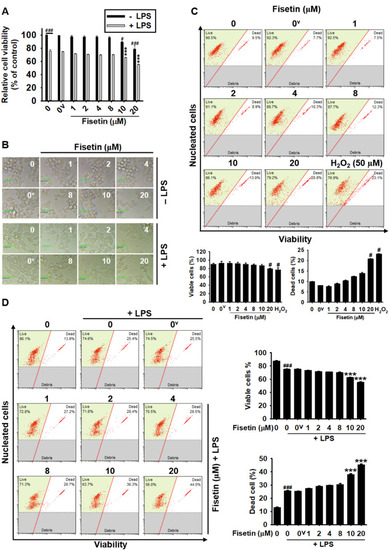
High concentrations of fisetin decreases the cell viability of RAW 264.7 macrophages. RAW 264.7 macrophages (1 × 105 cells/mL) were treated with fisetin (0–20 µM) for 24 h in the presence or absence of 500 ng/mL LPS. ( |
|
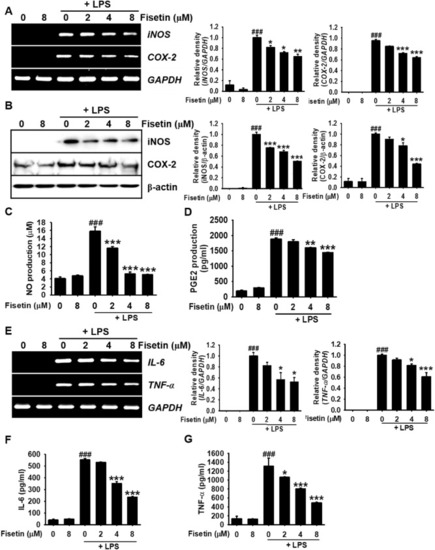
Fisetin decreases LPS-induced inflammatory mediator and cytokine levels in RAW 264.7 macrophages. RAW 264.7 macrophages (1 × 105 cells/mL) were treated with fisetin (0–8 µM) 2 h before treatment with 500 ng/mL LPS. ( |
|
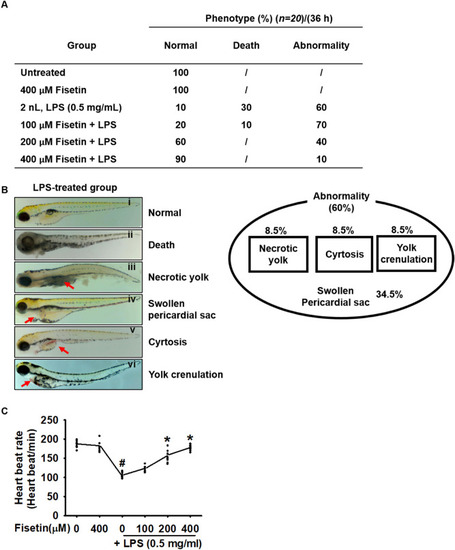
Fisetin attenuates LPS-induced mortality, abnormalities, and lowered heart rate in zebrafish larvae. Zebrafish larvae at 3 dpf ( |
|
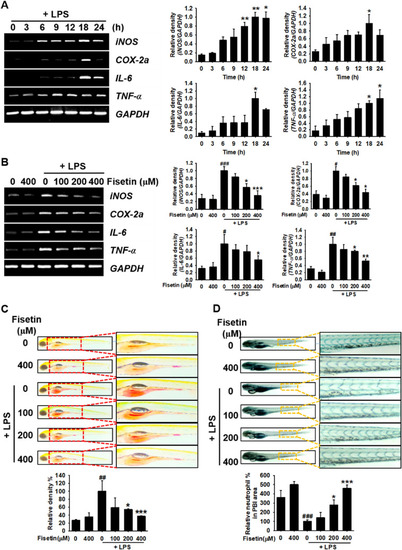
Fisetin inhibits LPS-induced inflammatory response in zebrafish larvae. Zebrafish larvae at 1 day post fertilization (dpf) were cultured in 0.003% PTU containing E3 embryo media. Briefly, 2 nL of 0.5 mg/mL LPS was microinjected into the yolk at 3 dpf. Zebrafish larvae were immediately immersed in E3 embryo media containing different concentrations of fisetin. ( |
|
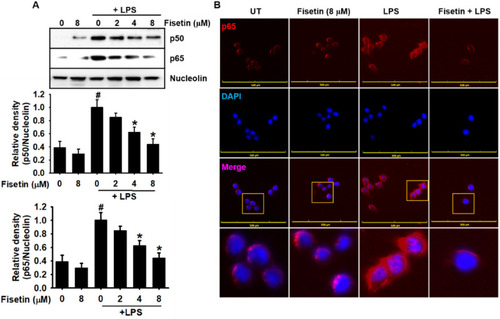
Fisetin inhibits LPS-induced nuclear translocation of NF-κB in RAW 264.7 macrophages. RAW 26.4.7 cells (1 × 105 cells/mL) were pretreated with fisetin 2 h before LPS treatment (500 ng/mL; 0.5 h). ( |
|
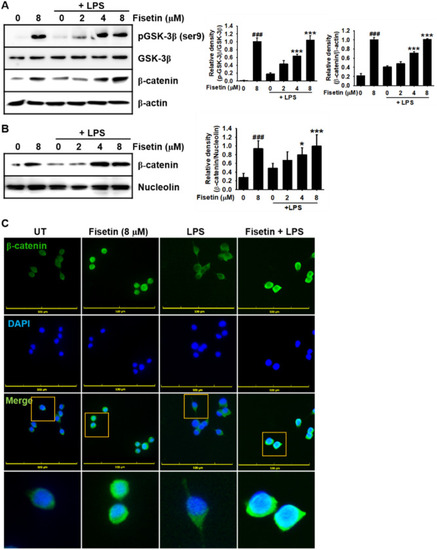
Fisetin promotes the expression of β-catenin and its nuclear translocation in LPS-stimulated RAW 264.7 macrophages. RAW 264.7 macrophages (1 × 105 cells/mL) were pretreated with fisetin 2 h prior to LPS treatment (500 ng/mL; 0.5 h). ( |
|
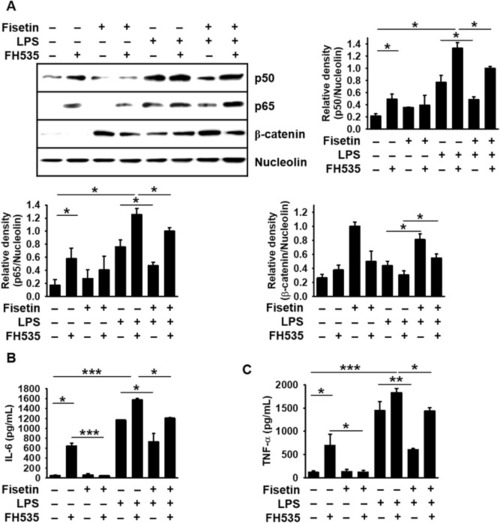
Fisetin inhibits LPS- or FH535-mediated IL-6 and TNF-α release by stimulating β-catenin-mediated NF-κB inactivation. RAW 264.7 macrophages (1 × 105 cells/mL) were incubated with 10 µM FH535 for 2 h prior to treatment with 8 μM fisetin and 500 ng/mL LPS. ( |
|
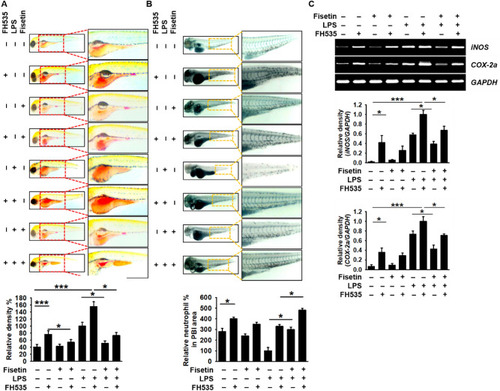
Fisetin inhibits LPS-induced inflammation and endotoxic shock by stimulating the β-catenin signaling pathway in zebrafish larvae. Zebrafish larvae at 3 dpf were pretreated with 10 µM FH535 for 2 h prior to LPS (2 nL of 0.5 mg/mL) microinjection. Then, the larvae were grown in E3 media containing 400 μM fisetin. ( |
|
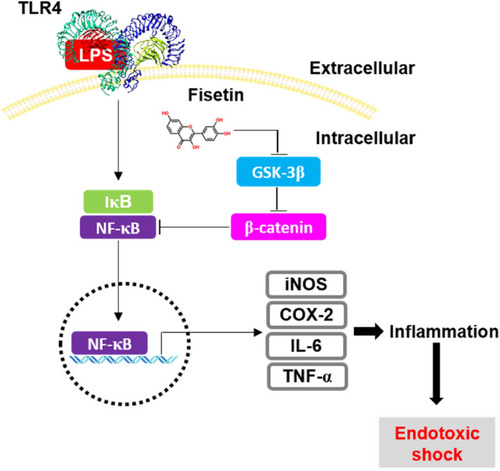
Fisetin inhibits GSK-3β-mediated NF-κB activation in the presence of β-catenin, leading to the inhibition of inflammation-induced septic shock. Once macrophages are exposed to high concentrations of the bacterial endotoxin, LPS, they initiate an inflammatory response and endotoxic shock by upregulating the expression of NF-κB-induced inflammatory genes, such as |