- Title
-
Carbofuran accelerates the cellular senescence and declines the life span of spns1 mutant zebrafish
- Authors
- Khan, A., Fahad, T.M., Akther, T., Zaman, T., Hasan, M.F., Islam Khan, M.R., Islam, M.S., Kishi, S.
- Source
- Full text @ J. Cell. Mol. Med.
|
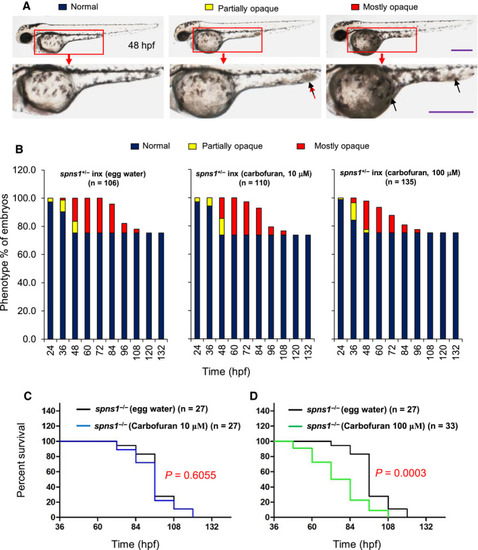
Carbofuran accelerated yolk opaqueness and SA‐β‐galactosidase activity in |
|
Carbofuran exacerbated the |
|
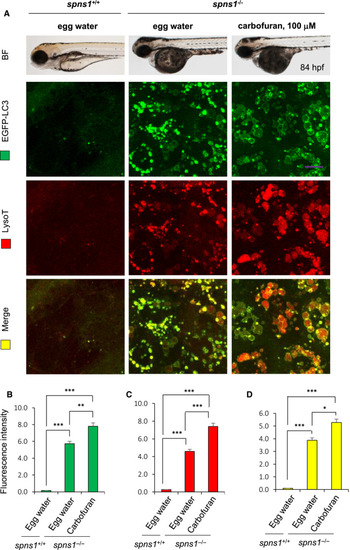
Carbofuran accelerated autolysosomal puncta formation in |
|
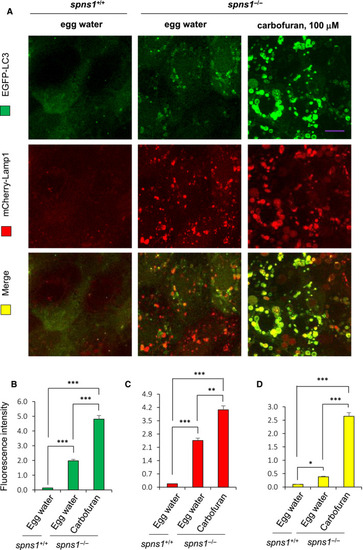
Carbofuran accelerated autolysosomal puncta formation in a double‐transgenic zebrafish line of |
|
RT‐PCR analysis to determine the effect of carbofuran on |
|
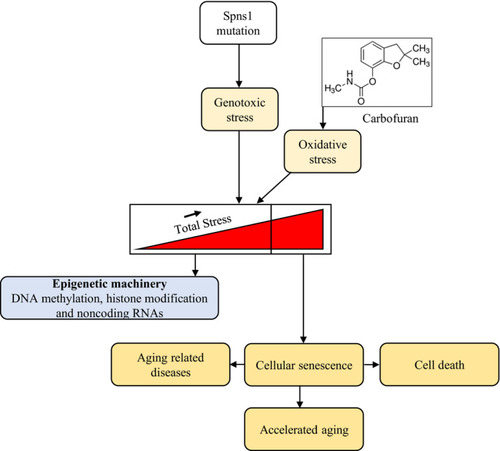
A proposed schematic model for the senescence and ageing effect of carbofuran under |