- Title
-
Tbx20 Induction Promotes Zebrafish Heart Regeneration by Inducing Cardiomyocyte Dedifferentiation and Endocardial Expansion
- Authors
- Fang, Y., Lai, K.S., She, P., Sun, J., Tao, W., Zhong, T.P.
- Source
- Full text @ Front Cell Dev Biol
|
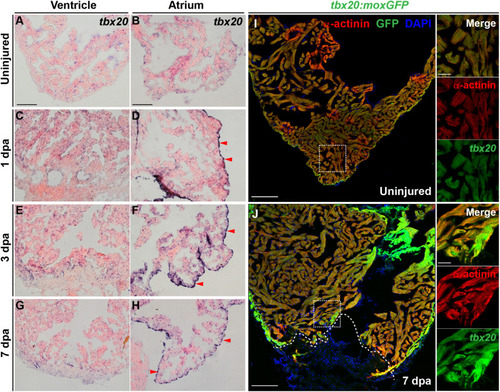
Cardiac injury triggers a localized increase in |
|
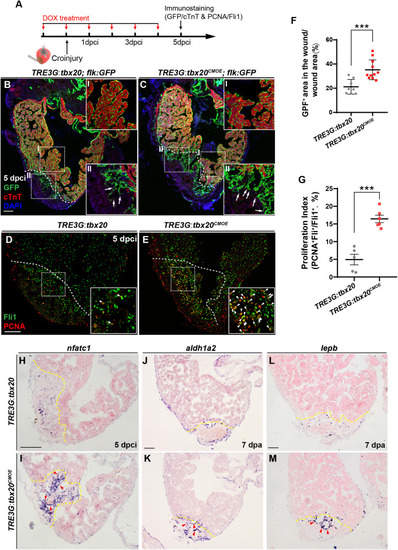
Myocardial |
|
|
|
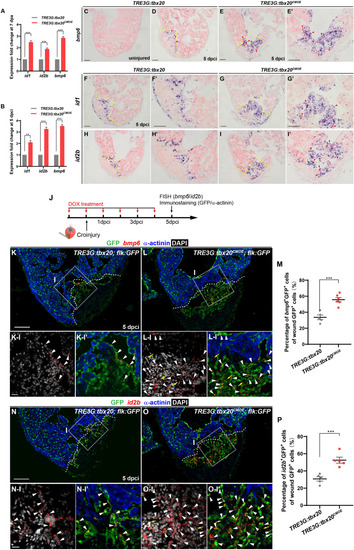
Myocardial |
|
Myocardial |
|
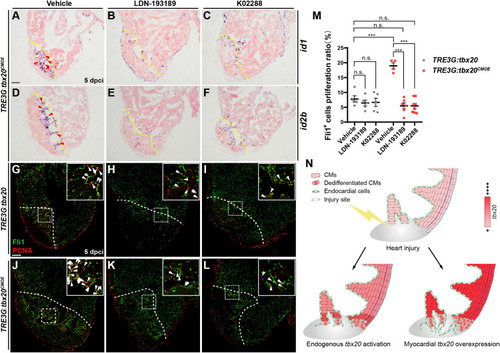
Myocardial Tbx20 mediates endocardial regeneration by activating Bmp6 signaling. |
|
Inhibition of Bmp6 signaling restricts endocardial cell proliferation activated by myocardial |