- Title
-
Redundant Trojan horse and endothelial-circulatory mechanisms for host-mediated spread of Candida albicans yeast
- Authors
- Scherer, A.K., Blair, B.A., Park, J., Seman, B.G., Kelley, J.B., Wheeler, R.T.
- Source
- Full text @ PLoS Pathog.
|
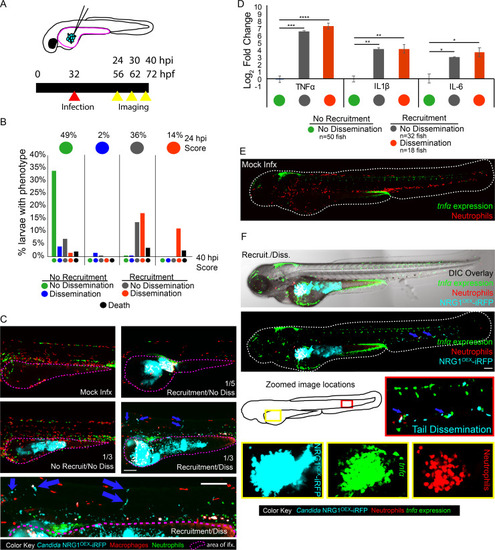
Larvae were infected with far-red fluorescent yeast-locked |
|
|
|
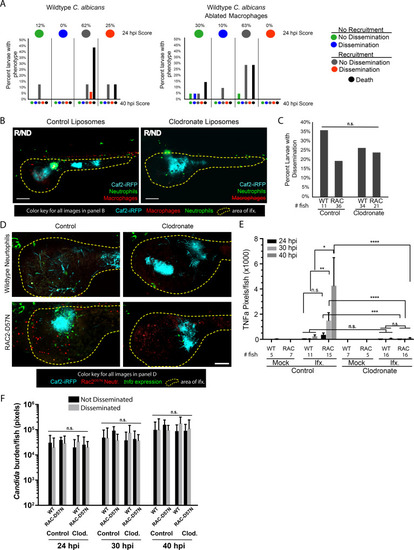
Rac2-D57N and AB sibling larvae were injected with control or clodronate liposomes mixed with a 10 kDa dextran conjugated with Cascade Blue in the caudal vein at 28 hpf. Larvae were infected with the yeast locked |
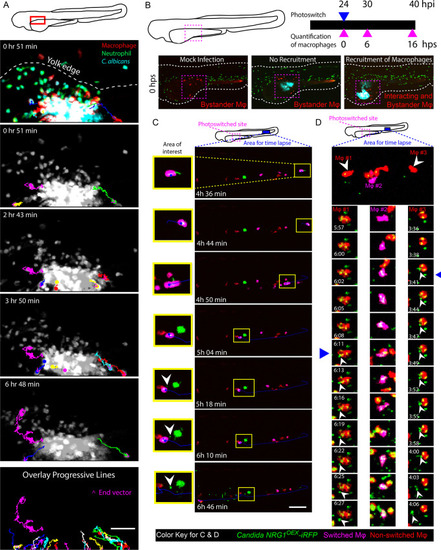
|
|
|
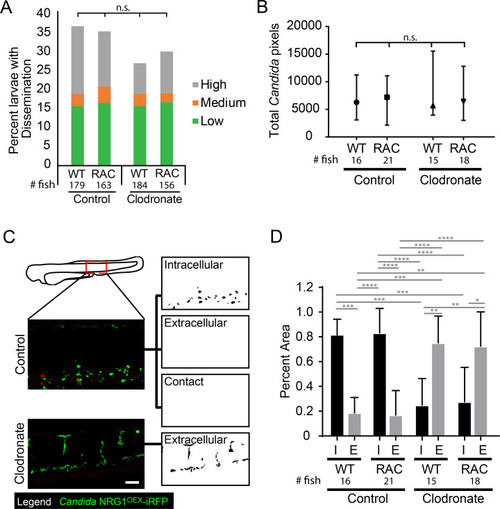
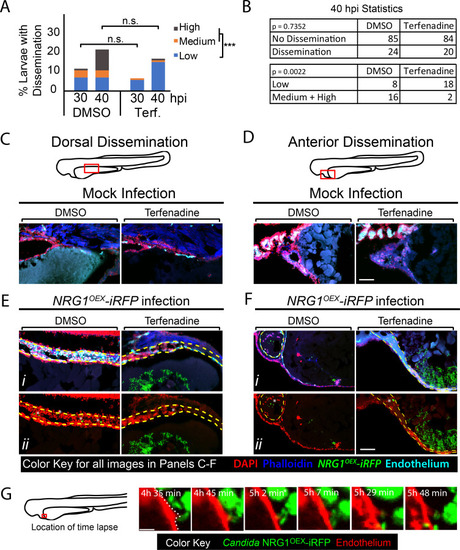
(A-G) |
|
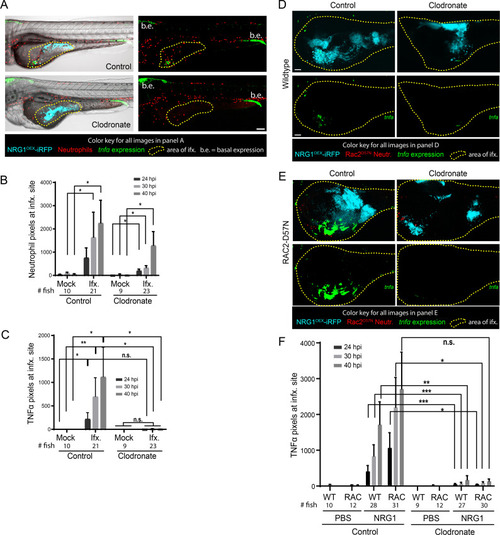
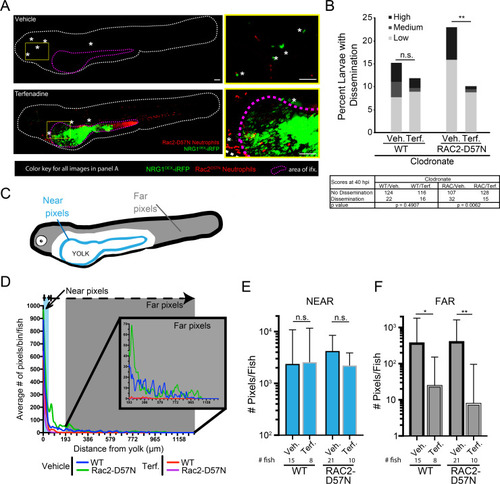
Rac2-D57N zebrafish were crossed with AB fish for neutrophil-deficient or wild type offspring. All larvae were injected with clodronate liposomes for macrophage ablation and bathed in vehicle (DMSO) or 2 μM terfenadine for blood flow blockade. Larvae were then infected with NRG1OEX-iRFP. (A) Representative Rac2-D57N larvae with dissemination either with (Vehicle) or without (Terfenadine) blood flow. The white line outlines the fish body, the magenta line indicates the yolk sac, and white asterisks denote yeast that are disseminated. The yellow box indicates an area with disseminated yeast that has been magnified at the right. Scale bar = 100 μm. (B) Percent larvae with scores of dissemination at 40 hpi, pooled from 6 experiments. Stats: Fisher’s exact test, * p ≤ 0.05. (C) Schematic of the scoring system for disseminated yeast. Yeast that were ≤25 pixels from the yolk sac edge were scored as “near” and yeast that were ≥55 pixels from the yolk sac edge were scored as “far”. (D) A frequency distribution histogram of the distance in pixels that yeast travel from the yolk sac edge sorted into single bins. Distances less than 5 pixels was omitted from analysis. Same fish quantified as in C and D. Pooled data from 54 larvae. (E) Total number of near yeast pixels per fish, shown as median and confidence interval (F) Total number of far yeast pixels per fish, shown as median and confidence interval. (D-F) Pooled from 6 experiments. Stats: Mann-Whitney, n.s. not significant, * p ≤ 0.05, ** p ≤ 0.01. |
|
|