- Title
-
The Diverse Roles of Phagocytes During Bacterial and Fungal Infections and Sterile Inflammation: Lessons From Zebrafish
- Authors
- Linnerz, T., Hall, C.J.
- Source
- Full text @ Front Immunol
|
Schematic illustration of the different delivery routes in larval zebrafish for pathogens. ( |
|
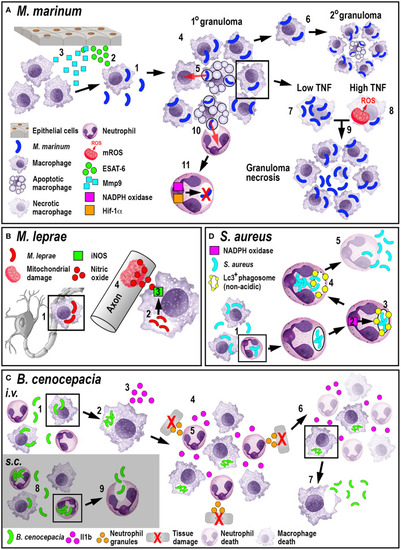
Schematic illustration of the phagocyte responses to the bacterial pathogens |
|
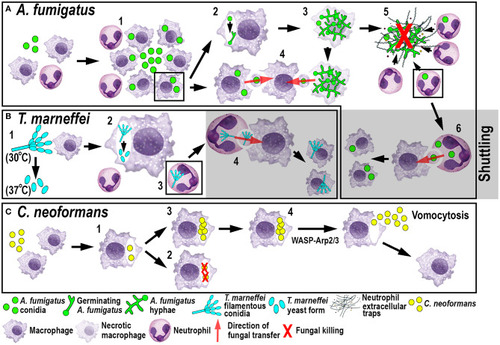
Schematic illustration of phagocyte responses to the fungal pathogens |
|
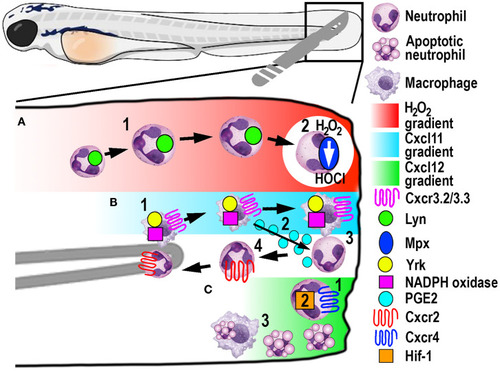
Schematic illustration of the signaling pathways and mechanisms that help control phagocyte migration and abundance during larval zebrafish acute tail fin injury. |