- Title
-
Activity of lumacaftor is not conserved in zebrafish Cftr bearing the major cystic fibrosis-causing mutation
- Authors
- Laselva, O., Erwood, S., Du, K., Ivakine, Z., Bear, C.E.
- Source
- Full text @ FASEB Bioadv
|
The processing defect of F507‐zCftr is fully rescued by low temperature correction at 27°C. A, HEK293 cells were transiently transfected with WT‐zCftr‐GFP. To evaluate glycosylation status, immunoblots show WT‐zCftr‐GFP control (−) and its sensitivity to endoglycosidase H (H) and peptide‐N‐glycosidase F (F). White arrowhead, complex glycosylated (Band C); gray arrowhead, core glycosylated (Band B). The zCftr‐GFP was detected with an antibody against GFP. B, HEK293 cells were transiently transfected with WT‐hCFTR‐GFP, F508del‐hCFTR‐GFP, WT‐zCftr‐GFP, or F507del‐zCftr‐GFP at 37°C (left) or 27°C (right) (n = 4). The zebrafish mutant recapitulates the primary defect in processing observed in the human mutant at 37°C. C, Immunoblots show F507del‐zCftr‐GFP protein exhibit partial processing to mature form, Band C at 37ºC, as evident in its resistance to endoglycosidase H (H) and sensitivity to peptide‐N‐glycosidase F (F). D, Bar graphs show the mean (±SEM) of the ratio (Band C)/(Band (C + B)) of the WT and F508del‐hCFTR‐GFP proteins plus WT and F507del‐zCftr‐GFP protein at 37 and 27°C (n = 4) (*** |
|
At low temperature, F507del‐zCftr generates a stable mature complex‐glycosylated protein. A, F508del‐hCFTR or F507del‐zCftr‐GFP was expressed in HEK293 cells. After 24 h incubation at 27°C, protein synthesis was inhibited by addition of CHX (0.5 mg/mL) and then the cells were shifted to 37°C to monitor thermal stability. Cells were collected after the indicated times for western blot analysis of whole cell extracts. B, The total amount of F508del‐hCFTR or F507del‐zCftr protein at each time point was quantitated and expressed relative to that at time 0 and calnexin loading (n = 4). CHX, cycloheximide; GFP, green fluorescent protein; hCFTR, human CFTR; HEK293, human embryonic kidney 293 |
|
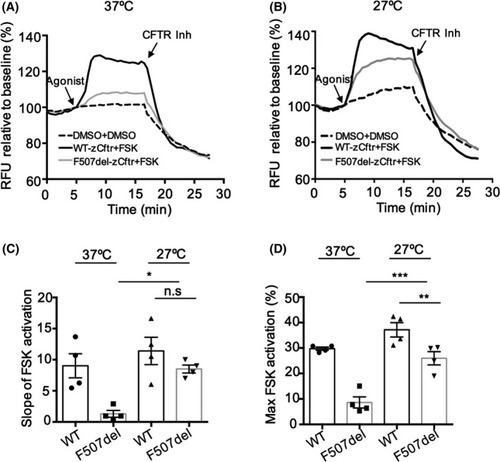
Activation of F507del‐zCftr exhibits full rescue after low temperature rescue. Functional analysis using the fluorometric imaging plate reader membrane depolarization assay of WT‐or F507del‐zCftr‐GFP in the presence of 0.1% DMSO or 10 μM of FSK in HEK293 cells at 37°C (A) or 27°C (B). After 10 min activation by FSK, CFTR Inhibitor (CFTRinh‐172, 10 μM) was added to deactivate CFTR. (C) Bar graph shows the mean (±SEM) of the slope of FSK activation at 37°C and 27°C (n = 4). (D) Bar graphs show the mean (±SEM) of maximal activation of CFTR after stimulation by FSK at 37 and 27°C (n = 4). (* |
|
WT‐zCftr and F507del‐zCftr channel activity can be potentiated by VX‐770. A, Functional analysis of WT‐zCftr‐GFP in HEK293 cells using FLIPR assay by 0.1% DMSO or 1 μM FSK in the presence or absence of 1 μM VX‐770, at 37°C. After 10 min activation by FSK, CFTR Inhibitor (CFTRinh‐172, 10 μM) was added to deactivate CFTR. B, Bar graph shows the mean (±SEM) of the slope of FSK activation at 37°C (n = 4). C, Representative traces of F507del‐zCftr‐GFP function following 24 h preincubation at 27°C and acute activation with 1 μM FSK ± 1 μM VX‐770 at 27°C. D, Bar graph shows the mean (±SEM) of the slope of FSK activation at 27°C (n = 4). (** |
|
At 37°C, zebrafish Cftr (F507del) exhibits negligible rescue of the processing defect with VX‐809. A, HEK cells were transiently transfected with F508del‐hCFTR‐GFP (left) and F507del‐zCftr‐GFP (right two panels), in the presence or absence of 3 μM VX‐809 at 37 or 27°C. Band C, fully processed, mature complex‐glycosylated CFTR; Band B, immature core‐glycosylated CFTR are indicated. Unlike the human mutant, there is no positive effect of VX‐809 on processing of the zebrafish mutant. B, Bar graphs show the quantification of immunoblots like those shown in A) for four biological repeats. The bars show mean (±SEM) of the ratio C/(C + B) for the human and zebrafish mutants. C, Representative traces (membrane depolarization assay) for F508del‐hCFTR‐GFP channel activation and potentiation, with or without VX‐809 pretreatment. In these experiments, CFTR‐mediated depolarization was inhibited with the addition of CFTRinh‐172 (10 µM). D, Unlike the human mutant, there is no effect of VX‐809 on activated and potentiated F507del‐zCftr‐GFP channel function following chronic treatment with 3 μM VX‐809. E, Bar graph shows the mean (±SEM) of the initial slope after potentiation at 37°C (n = 4). Calnexin (CNX) was used as a loading control. (** |