- Title
-
Reversion of tumor hepatocytes to normal hepatocytes during liver tumor regression in an oncogene transgenic zebrafish model
- Authors
- Li, Y., Agrawal, I., Gong, Z.
- Source
- Full text @ Dis. Model. Mech.
|
CreER/xmrk double-transgenic zebrafish to trace tumor hepatocytes. (A) Schematics of the transgene constructs for CreER and xmrk transgenic zebrafish. The xmrk construct contains the Tet-on-regulated xmrk under the hepatocyte-specific fabp10 promoter, with the skin promoter krt4 driving GFP as the selection marker. In the CreER construct, the hepatocyte-specific promoter fabp10 drives the floxed EGFP followed by DsRed. CreERT2 is controlled by the Tet responsive element (TRE). In the presence of Dox, rtTA will be activated, which in turn activates the xmrk oncogene to transform hepatocytes into tumor hepatocytes and also induce the expression of CreERT2. Subsequent addition of 4-OHT will activate CreERT2 to cause loxPrecombination and thus irreversibly label tumor cells with RFP expression. (B) Liver-specific induction of CreERT2 expression. Whole-mount in situ hybridization using anti-sense and sense probes against Cre mRNA was carried out in CreER and CreER/xmrk larvae at 4 dpf. No signal was detected with the sense probe in both CreER and CreER/xmrk larvae. Using the antisense probe, no expression of CreERT2 was observed in the liver of CreER larvae, either in the absence or presence of Dox (top panels). No expression of CreERT2 was observed in the liver of CreER/xmrk larvae in the absence of Dox (the far-left bottom panel). Liver-specific induction of CreERT2 expression was only observed in the liver of CreER/xmrk larvae in the presence of Dox (middle and far-right bottom panels). Livers are outlined in dash lines (L). Non-specific hybridization signals were observed in the regions of swimbladder (SB) and esophagus (E) in some samples. (C) Switch of fluorescent protein expression in livers in CreER/xmrk larvae following Dox and 4-OHT treatments at 6 dpf. The livers of CreER larvae retained GFP expression in all the four treatment conditions. The livers of CreER/xmrk larvae had GFP expression in both Dox– 4-OHT– and Dox– 4-OHT+ treatment conditions, and no leaky expression of RFP was observed (the far-left bottom panel). Leaky CreER activity was observed in the Dox+ 4-OHT– treatment condition. Dox+ 4-OHT+ treatment caused the uniform conversion of the liver for RFP expression in the CreER/xmrk fish. Livers are outlined in dash lines. |
|
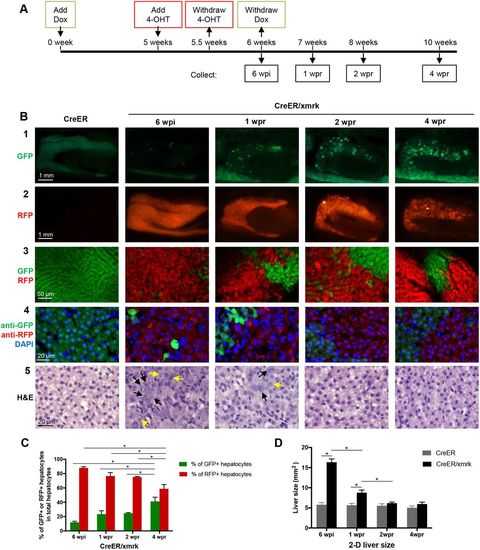
Tracing liver tumor cells during tumor regression in adult fish. (A) Flowchart of fish treatment and sample collection. CreER and CreER/xmrk adult fish (4 mpf) were treated with Dox for 6 weeks to induce HCC. 4-OHT was added at 5 wpi (weeks post-induction) for 3 days to cause loxPrecombination for marking the tumor cell lineage with RFP expression. Fish were collected at 6 wpi, 1 wpr (weeks post-regression), 2 wpr and 4 wpr for various assays. (B) Fluorescent protein expression and histological analyses of livers. Rows 1 and 2 are representative images of gross observation of GFP and RFP in the livers under a stereomicroscope. Row 3 shows representative confocal images of the fresh liver tissue immersed in phosphate buffer to show the re-establishment of the two-cell hepatic plate structure from tumor-reverted hepatocytes during tumor regression. Row 4 shows representative images of fixed liver sections for GFP expression, RFP expression (stained with anti-RFP antibody) and nuclear staining by DAPI for quantification of cell number. Row 5 shows representative images of H&E staining. Black arrows, large and irregular nuclei; yellow arrows, prominent nucleoli. (C) Quantification of percentages of GFP+ hepatocytes and RFP+ hepatocytes in total hepatocytes based on liver sections from panel B row 3 (n=10). (D) Quantification of 2D liver size based on the left lateral view of dissected fish as shown in row 2 of panel B (n=10/group). *P<0.05. |