- Title
-
Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes
- Authors
- Paatero, I., Casals, E., Niemi, R., Özliseli, E., Rosenholm, J.M., Sahlgren, C.
- Source
- Full text @ Sci. Rep.
|
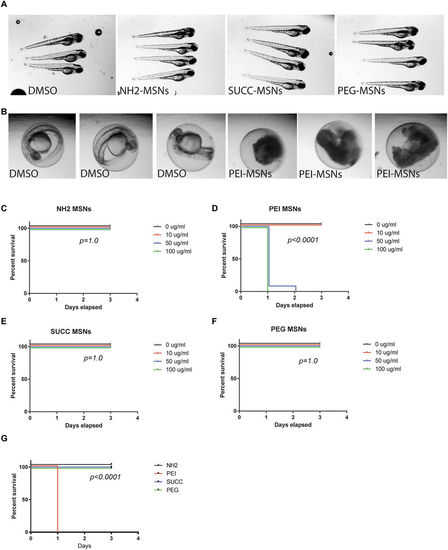
Surface functionalization affects overall toxicity of MSNs. (A) Zebrafish embryos at 72 hpf treated with the DMSO vehicle, NH2-, SUCC- and PEG-MSNs at a concentration of 50 μ/ml. Treatment was initiated at 8hpf. The embryos are anesthesized and mounted in 2%methyl cellulose. (B) Zebrafish embryos at 48 hpf treated the DMSO vehicle or 50 μg/ml PEI-MSNs. Treatment was initiated at 8hpf. (C–F) Percentage of survival of embryos at 24hpf, 48 hpf and 72hpf upon treatment with (C) NH2-, (D) PEI-, (E) SUCC- and (F) PEG-MSNs at denoted concentrations. The treatment was initiated at 8hpf. N = 24 embryos/treatment, except in PEI-MSN 10 μg/ml (n = 25) and SUCC-MSN 100 μg/ml (n = 25). Each embryo was followed throughout the experiment and score live or dead on each day (G) Comparison of survival between nanoparticles at 100 μg/ml concentration. |
|
Dechorionation sensitizes embryos for MSN induced toxicity. (A) Images of dechorionated zebrafish embryos at 96 hpf after treatment with NH2. PEI, SUCC and PEG.-MSNs. The treatment was initated at 24hpf. (B–E) Percentage of survival of dechorionated embryos at 48 hpf, 72hpf and 96 hpf upon treatment with (B) NH2, (C) PEI, (D) SUCC and (E) PEG MSNs at denoted concentrations. The treatment was initated at 24hpf. N = 24 embryos/treatment, except in PEI-MSN 0 μg/ml (n = 25), SUCC-MSN 0 μg/ml (n = 23), SUCC-MSN 10 μg/ml (n = 23) and SUCC-MSN 100 μg/ml (n = 25). Each embryo was followed throughout the experiment and score live or dead on each day (F) Comparison of survival between MSNs at 100 μg/ml concentration. |
|
Surface functionalization affects aggregation of MSNs on the embryo skin. The uptake of the nanoparticles from the aquaeous medium into the embryo was analyzed by mounting anesthesized living embryos into agarose and imaged using confocal microscopy (Leica SP5 Matrix). A transgenic strain (kdrl:EGFP) expressing a GFP labeled vasculature were used 24hpf embryos were incubated 48 h with the highest tolerated dose of the different nanoparticles e.g 100 μg/ml NH2-, SUCC- and PEG-MSNs, and 10 ug/ml of PEI-MSNs. DMSO treated embryos were used as controls and show background fluoresence (A). Maximum Z-projections are shown. The white arrows mark nanoparticle clusters on the superficial layer of the embryo. |
|
Surface functionalization affects uptake of MSNs. The uptake of the nanoparticles from the aquaeous medium into the embryo was analyzed by mounting anesthesized living embryos into agarose and imaged using confocal microscopy (Leica SP5 Matrix). A transgenic strain (kdrl:EGFP) expressing a GFP labeled vasculature were used. 24hpf embryos were incubated for 48 h with the highest tolerated dose of the different nanoparticles e.g 100 μg/ml NH2-, SUCC- and PEG-MSNs, and 10 μg/ml of PEI-MSNs. DMSO treated embryos were used as controls and show background fluorescence (A). Ortohogonal views of the 3D confocal data sets are shown to visualize fluorescence deep inside the embryo. Panels are pseudocolored for clearer visualization of nanoparticle fluorescence in the brain. Calibration bar in panel 6A indicates relation between colour and intensity values. White arrows mark nanoparticle clusters inside the embryo brains. Scale bar is 100 µm. |