- Title
-
The LRR receptor Islr2 is required for retinal axon routing at the vertebrate optic chiasm
- Authors
- Panza, P., Sitko, A.A., Maischein, H.M., Koch, I., Flötenmeyer, M., Wright, G.J., Mandai, K., Mason, C.A., Söllner, C.
- Source
- Full text @ Neural Dev.
|
Zebrafish islr2 is expressed in RGCs and is necessary for complete retinal axon midline crossing. a Time-course analysis of islr2 mRNA expression in zebrafish embryos and larvae. At 36 hpf, the first time point at which the optic chiasm is established, islr2 is already present in RGCs (arrowheads). At 48 hpf the RGC layer is evenly populated by neurons expressing islr2. At 72 hpf we detected a slight decrease in the islr2 mRNA level and the presence of a subset of islr2-positive amacrine cells. Dashed outlines highlight retinas in ventral views. Upper row: ventral view. Lower row: lateral view. Anterior is to the left. b Schematic of the islr2 sa82 TILLING allele. The mutant ORF harbors a premature stop codon caused by a C-to-T mismatch, ultimately leading to a truncation of the LRR-CT domain. c . islr2 mutant larvae show ipsilateral retinal projections. First row: dorsal view of a wild-type larva at 5 dpf. The optic nerves from each eye (green and magenta) cross the midline to innervate the contralateral optic tectum (areas delimited by dashed lines). Second and third row: two homozygote mutant animals, displaying ectopic innervation of the ipsilateral tectum (asterisks). The larva in row 2 additionally shows a thinner optic nerve (arrow), while the individual in row 3 has optic nerves with normal width. All pictures are dorsal views and maximum intensity projections of confocal Z-stacks. Anterior is to the left. Scale bar: 50 μm. d Direct relationship between optic nerve width (brackets) and ipsilateral misrouting of retinal axons (arrowheads) at the chiasm of islr2 mutant larvae. Homozygous mutant individuals with normal optic nerve (second column) display a higher number of ipsilateral fibers, compared to mutants with thinner nerves. Single optical sections. Ventral views. Anterior is to the top. ON: optic nerve. Scale bar: 50 μm. d' Phenotype distribution in islr2 mutants compared to siblings. Joint analysis of two clutches of animals showing mostly unaltered optic nerve width. Mutant larvae (black bars) display a higher number of ectopic neurites in the ipsilateral optic tectum, compared to controls (grey bars) and to larvae with thinner optic nerves (compare to d''). The innervation area corresponding to the contralateral optic tectum was unaffected in mutants compared to siblings. CL: contralateral. d'' Joint analysis of two clutches where larvae with thinner nerves were identified. Only a subset of mutant animals (black bars) displays ipsilateral innervation of the optic tectum, while in many mutants these neurites are undetectable, likely because of the extreme reduction in retinal fiber number. In most mutant animals the innervation of the contralateral optic tectum is reduced, suggesting that thinning of the optic nerve and ipsilateral projections are independent phenotypes. CL: contralateral EXPRESSION / LABELING:
PHENOTYPE:
|
|
Chronological progression of the islr2−/− phenotype. Anterogradely-labeled retinal projections of islr2 mutant embryos and larvae compared to control siblings. At 40 and 48 hpf growth cones can be observed in the proximity of the midline in mutant embryos, while these seem to have proceeded to the optic tract in wild-type siblings. Additionally, axonal extension defects can be recognized in mutants compared to controls (40 hpf, 48 hpf, 72 hpf, islr2−/−, first column). From 48 hpf, ipsilateral neurites (arrow) are visible in mutant embryos and can be traced back to the chiasm. As expected, 5 dpf mutant larvae displayed ectopic innervation of the ipsilateral optic tectum (asterisks). At this stage, islr2−/− larvae show misrouted fibers at the chiasm (arrowheads). All pictures are maximum intensity projections of confocal Z-stacks. Anterior is to the top. Scale bar: 50 μm |
|
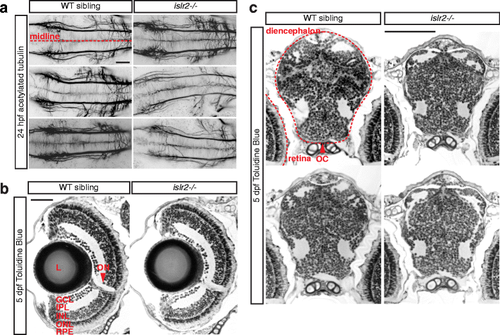
Zebrafish islr2 mutants do not display widespread axon guidance defects and are morphologically normal. a Acetylated tubulin staining of 24 hpf embryos showing central axon tracts at the level of the medial longitudinal fasciculus (MLF). Thinner axon bundles were observed only in a subset of islr2 mutant animals. The general spacing and directionality of nerve fibers was unaltered compared to controls, indicating absence of guidance defects in these axons. Maximum intensity projections of confocal Z-stacks. Dorsal views. Anterior is to the left. Scale bar: 50 μm. b Transverse sections through the retina of 5 dpf islr2 mutants. Normal size, gross anatomy and layering of the retina were observed in islr2 mutants. RGC number may be decreased in some islr2−/− larvae. L: lens. GCL: ganglion cell layer. IPL: inner plexiform layer. INL: inner nuclear layer. ONL: outer nuclear layer. RPE: retinal pigmented epithelium. ON: optic nerve. Scale bar: 50 μm. c Transverse sections at the level of the optic chiasm in islr2 mutants and siblings. The chiasmatic and ventral diencephalic tissue appears normal in size and patterning, suggesting that the axon guidance phenotype of islr2 larvae does not originate from mispatterning effects. OC: optic chiasm. Scale bar: 100 μm |
|
Loss of Islr2 leads to axon routing errors at the optic chiasm and tract in mice. E16.5 Islr2 mutant mice display expanded chiasms along the antero-posterior axis (brackets in b and c, compared to a). In addition, a greater number of axons enter the opposite optic nerve with retinopetal directionality (arrows in b and c). Although no clear increase in the number of ipsilaterally-projecting fibers relative to the contralateral projection can be observed, other defects were identified along the postcrossing route of RGC axons. Defasciculation effects in the optic tract were observed both at ventral (b' and c')and dorsal locations (b'' and c'', where many axons stray from their normal course (arrowheads). Arrowheads in b' and c' indicate axons departing rostrally from the chiasm, a common phenotype observed in situations when guidance through the chiasm is impaired. I: ipsilateral. C: contralateral. ON: optic nerve. OC: optic chiasm. OT: optic tract. Scale bars: 500 μm (a-c), 200 μm (a'-c' and a''-c''). |
|
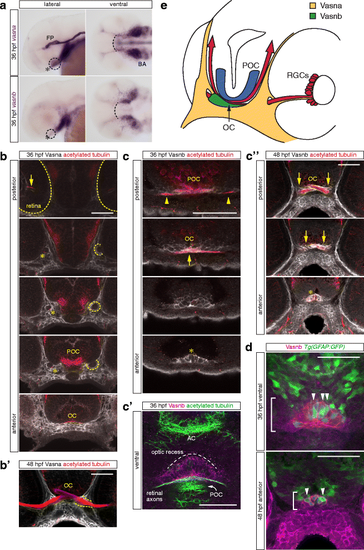
Vasna and Vasnb are specifically expressed in the retinal axon pathway at 36 and 48 hpf. a Whole-mount in situ hybridization of vasna and vasnb mRNA reveals the expression of these genes (dashed circles) in the region of the RGC axon commissure (asterisk). At 36 hpf, when the optic chiasm is first established, vasna is highly expressed in the floor plate (FP) and prospective branchial arches (BA). Additionally, vasna marks mesenchymal tissue associated with the retinal axon pathway. vasnb expression appears to border anteriorly the vasna-positive ventral domain (dashed arc). vasnb mRNA level is relatively low in this region. Lateral and ventral views. Anterior is to the left. FP: floor plate. BA: branchial arches. b A polyclonal anti-Vasna antibody shows Vasna localization to the membrane of mesenchymal cells surrounding ventro-laterally the retinal projection. At 36 hpf, Vasna-positive cells of the primitive optic artery (arrow) are already associated to retinal axons in the eye (dashed outlines). These two cell types stay in contact until retinal axons enter the diencephalic floor. At this point, axons lose contact with the vasculature and encounter the opening of a tunneling structure, marked by Vasna on its surface (dashed circles). Axons always associate with the ventral side of this channel (asterisks). At its exit, retinal axons are brought in the neighborhood of the prospective optic chiasm, which is bordered ventrally by Vasna-positive mesenchymal tissue. Each picture is a single optical section from a Z-stack encompassing antero-posteriorly the whole course of retinal axons. Frontal views. POC: postoptic commissure. OC: optic chiasm. Scale bar: 50 μm. b' At 48 hpf, many axons have extended through the lateral Vasna-positive tunnels (dashed lines). Qualitatively the expression pattern of Vasna does not change from 36 hpf. Frontal view. OC: optic chiasm. Scale bar: 50 μm. c A polyclonal antibody against Vasnb marks a subset of midline cells in the ventral diencephalon. As soon as retinal axons enter the diencephalon, they are in contact with laterally-positioned Vasnb-positive cells (arrowheads). At the chiasmatic midline (arrow), retinal axons are surrounded by Vasnb-positive membranes. Vasnb-expressing cells populate the anterior-most ventral diencephalic tissue, until the forebrain ventricle opens. This location is consistent with the reported position of the glial knot (asterisk). Single optical sections through the ventral diencephalon, posterior to anterior, are shown. Frontal views. POC: postoptic commissure. OC: optic chiasm. Scale bar: 50 μm. c' Ventral view of an embryo stained for Vasnb and acetylated tubulin. The preoptic area, between the optic recess and the chiasm is populated by cells expressing Vasnb. Vasnb-positive cells enwrap retinal axons. The picture is a Z-projection of 4 consecutive optical sections. Anterior is to the top. AC: anterior commissure. POC: postoptic commissure. Scale bar: 50 μm. c'' At 48 hpf, Vasnb is still detected on cells associated to the optic nerves (arrows) and in the glial knot (asterisk). From this stage Vasnb is clearly located on membranes wrapping the optic nerves in ventral positions along their course inside the brain. Each picture is a single optical section through the preoptic area. Frontal views. OC: optic chiasm. Scale bar: 50 μm. d A subset of Vasnb-positive cells in the knot region (brackets) expresses the transgenic glial marker Tg(gfap:GFP) (arrowheads). Single optical sections from confocal Z-stacks. Ventral and anterior views. Scale bars: 50 μm. e Tridimensional model of the retinal projection in zebrafish embryos at about 36 hpf. Retinal axons (red) are always in contact with Vasna-positive cells (yellow). Their ventral diencephalic course is shaped anteriorly by the Vasnb expression domain (green). The POC is shown in blue and borders the RGC axons caudally. OC: optic chiasm. POC: postoptic commissure. RGCs: retinal ganglion cells |
|
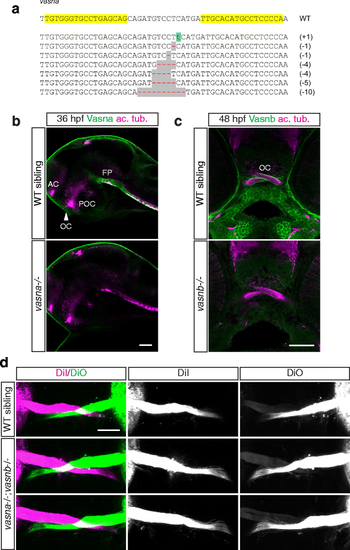
vasna/vasnb double mutants do not display overt axon guidance defects at the optic chiasm. a Alleles generated by targeted mutagenesis of the vasna locus, using TALEN technology. All alleles are followed by premature stop codons, leading to the translation of a short and functionally inactive protein. Yellow highlights show the genomic DNA target sequence for the engineered nucleases. b vasna alleles generated by targeted mutagenesis display complete loss of Vasna protein in homozygosity. AC: anterior commissure. FP: floor plate. OC: optic chiasm. POC: postoptic commissure. Single optical sections from confocal Z-stacks. Lateral views. Anterior is to the left. Scale bar: 50 μm. c The vasnb sa6027 TILLING allele leads to complete loss of function, as suggested by the absence of Vasnb immunoreactivity. Single optical sections from confocal Z-stacks. Frontal views. Scale bar: 50 μm. d 5 dpf vasna/vasnb double mutant larvae do not display strong axon guidance phenotypes at the optic chiasm. Ventral views of anterogradely-labeled optic nerves at the optic chiasm. Anterior is to the top. Scale bar: 50 μm |