- Title
-
A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production
- Authors
- Devireddy, L.R., Hart, D.O., Goetz, D.H., and Green, M.R.
- Source
- Full text @ Cell
|
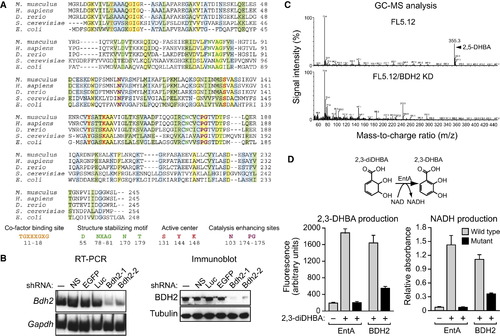
Identification of a Murine Homolog of Bacterial EntA that Mediates 2,5-DHBA Synthesis (A) Multiple sequence alignment of EntA/BDH2 homologs. Identical residues between all five proteins are indicated in yellow, conserved residues in green, and semiconserved residues in blue. (B) RT-PCR (left) and immunoblot (right) analysis monitoring murine Bdh2 expression in FL5.12 cells treated with one of two unrelated shRNAs targeting Bdh2 or a control nonsilencing (NS), enhanced green fluorescent protein (EGFP), or luciferase (Luc) shRNA. (C) GC-MS analysis of the eluted 24p3 sample prepared from parental FL5.12 cells or FL5.12 cells expressing a Bdh2 shRNA. (D) Wild-type and mutant bacterial EntA and mouse BDH2 proteins were analyzed for their ability to convert 2,3-diDHBA to 2,3-DHBA (left) or NAD to NADH (right). Error bars indicate the SD. Mutant EntA/BDH2 proteins were expressed at comparable levels to the wild-type proteins (Figure S2C). See also Figure S2. |
|
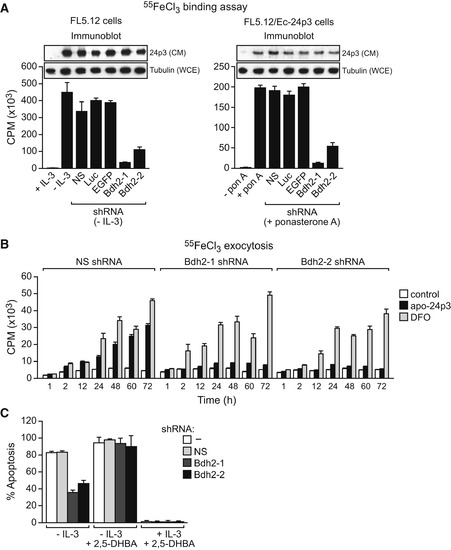
BDH2 Is Required for 24p3-Mediated Iron Transport and Apoptosis (A) Iron-binding assay. Left: FL5.12 cells were treated in the presence or absence of IL-3, and with a Bdh2 or control shRNA, and the iron-binding ability of 24p3 in the CM was monitored as described in Figure 1B. Inset: immunoblot analysis monitoring 24p3 levels in the CM and tubulin levels in the whole-cell extract (WCE). Right: FL5.12/Ec-24p3 cells were treated in the presence or absence of ponasterone A. Error bars indicate the SD. (B) Iron exocytosis assay. 55FeCl3-loaded FL5.12 cells were either untreated (control) or treated with apo-24p3 or DFO, and the presence of 55Fe in the medium was quantified. Error bars indicate the SD. (C) Apoptosis assay. FL5.12 cells cultured in the presence or absence of IL-3, with or without 2,5-DHBA, and expressing a NS or Bdh2 shRNA were analyzed for apoptosis by annexin V-FITC staining. Error bars indicate the SD. |
|
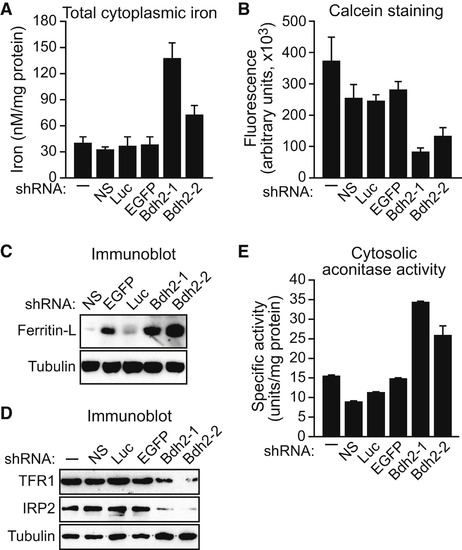
BDH2 Is Required to Maintain Normal Cytoplasmic Iron Levels FL5.12 cells expressing a Bdh2 or control shRNA were analyzed for total cytoplasmic iron levels by colorimetric analysis (A), for free cytoplasmic iron concentration by fluorescence calcein assay (B), for ferritin-L (C) and TFR1 and IRP2 (D) levels by immunoblot analysis, and for cytosolic aconitase activity (E). Error bars indicate the SD. See also Figures S3A–S3D and S4. |
|
Requirement of Vertebrate EntA Homologs for Heme Synthesis (A) MEL cells expressing a NS or Bdh2 shRNA were differentiated. After labeling with 55Fe, cells were lysed, and heme was extracted and analyzed for 55Fe incorporation. Error bars indicate the SD. (B) MEL cells were differentiated as described in (A), and after cell lysis, hemoglobin levels were determined by benzidine reaction. Hemoglobin content was expressed relative to that in untreated MEL cells cultured in the absence of dimethyl sulfoxide. Error bars indicate the SD. (C) MEL cells were differentiated as described in (A), and α- and β-globin mRNA levels were monitored by RT-PCR. The levels of Bdh2 and Gapdh were monitored as controls. (D) RT-PCR analysis. The bdh2 MO was injected into one-cell-stage embryos, and 24 hr later, RNA was extracted and analyzed by RT-PCR. Treatment with the bdh2 MO resulted in retention of the entire intron (upper band) as well as activation of a cryptic splice site that resulted in partial intron retention (lower band). (E) Phenotypic analysis of control MO- and bdh2 MO-injected embryos at 72 hr postfertilization (hpf). Bdh2-depleted embryos lack hemoglobinized erythrocytes, as evidenced by the absence of red color (arrow). (F) Whole-mount o-dianisidine staining of control MO and bdh2 MO-injected embryos at 72 hpf. Shown are lateral (top) and ventral (bottom) views of the anterior region of embryos. Control MO-injected embryos show positive o-dianisidine staining at the duct of Cuvier (DC) and the heart (H), which is markedly reduced in bdh2 MO-injected embryos. Right: quantification of the percent embryos with normal o-dianisidine staining. (G) Blood cells were isolated from control or bdh2 MO-injected embryos and analyzed for hemoglobinization by o-dianisidine staining. (H) Whole-mount o-dianisidine staining of embryos (72 hpf) injected with a control MO or bdh2 MO, or bdh2 MO-treated embryos coinjected with a bdh2 mRNA. (I) RT-PCR (left) and in situ hybridization analysis (right) for hbae1 and hbae3 expression in control and bdh2 MO-injected embryos. See also Figures S4E and S6. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Additional Experiments, Related to Figure 2 (A) Expression of Bdh2 in mouse adult tissue and embryos. RT-PCR analysis monitoring expression of Bdh2 or, as a loading control, Gapdh. (B) Identification of putative EntE and EntF homologs in mouse, human and zebrafish. Multiple sequence alignment of murine, human, zebrafish and bacterial homologs of EntE (left) and EntF (right). Regions of identity are indicated in gray. Only partial sequence of the 1293 amino acid bacterial EntF protein is shown. (C) SDS-PAGE analysis of purified recombinant wild-type and mutant murine BDH2 and bacterial EntA proteins. Purified recombinant wild-type and mutant BDH2 and EntA proteins were fractionated on an SDS-polyacrylamide gel and detected by Coomassie blue staining. Molecular weight markers are shown on the right, in kDa. |
|
Requirement of BDH2 for Intracellular Iron Homeostasis in Mouse mIMCD Kidney Cells, Related to [Figure 4], [Figure 5] and [Figure 6] (A) RT-PCR analysis monitoring expression of Bdh2 or, as a loading control, Gapdh in mIMCD cells treated with one of two unrelated shRNAs targeting Bdh2 or a control enhanced green fluorescent protein (EGFP), luciferase (Luc) or non-silencing (NS) shRNA. The results confirm that the two Bdh2 shRNAs efficiently knock down Bdh2 expression in mIMCD cells. (B-E) Cytoplasmic iron measurements. mIMCD cells expressing a Bdh2 or control shRNA were analyzed for total cytoplasmic iron levels by colorimetric analysis (B), for free cytoplasmic iron concentration by a fluorescence calcein assay (C), for ferritin-L levels by immunoblot analysis (D), and for ROS by fluorometric analysis of CDCF-DA fluorescence (E). Error bars indicate SD. The results demonstrate, consistent with our findings in FL5.12 cells, that cytoplasmic iron levels are elevated in BDH2 KD mIMCD cells. (F-J) Mitochondrial iron measurements. mIMCD cells expressing a Bdh2 or control shRNA were analyzed for total mitochondrial iron levels by colorimetric analysis (F), for RPA (G) and RPAC (H) fluorescence by fluorometry, for DHR 123 oxidation in the presence or absence of H2O2 by flow cytometry (I), and for mitochondrial aconitase levels (J). Error bars indicate SD. The results demonstrate, consistent with our findings in FL5.12 cells, that mitochondrial iron levels are decreased in BDH2 KD mIMCD cells. |
|
A Putative Yeast Homolog of Bacterial EntA Has Functional Similarities to Mammalian BDH2, Related to [Figure 4] and [Figure 7] (A) PCR analysis confirming the deletion of the yeast YIR035C gene and replacement with a KanMX gene cassette. The positions of the PCR primer-pairs are indicated in the schematic diagram, below. (B) Growth of wild-type and yir035cΔ strains. (Top) Five-fold serial dilutions were plated onto CSM dextrose in the absence or presence of the iron (Fe2+) chelator ferrozine (1 mM), and plates were incubated at 30°C for 1 day. (Bottom) Serial dilutions were plated onto CSM dextrose containing ferrozine (1 mM) in the absence or presence of 250 μM FeSO4. The results show that in medium containing low iron a yeast strain lacking YIR035C displays a growth defect, which is alleviated by addition of iron to the medium. (C) Control experiments showing the growth of two yeast strains deficient in genes that have well-characterized roles in iron utilization, FET3 and MRS3/4. Five-fold serial dilutions were plated onto CSM dextrose, or CSM dextrose containing ferrozine (1 mM) in the absence or presence of 250 μM FeSO4. The results show that the fet3Δ and mrs3/4Δ strains have an iron-dependent growth defect similar to that of the yir035cΔ strain. (D) Cytosolic aconitase activity was monitored in wild-type or yir035cΔ strains grown in CSM dextrose or CSM dextrose containing ferrozine (1 mM). The results show that the yir035cΔ strain has reduced cytoplasmic aconitase activity, which is greatly exacerbated in medium containing low iron. (E) Heme synthesis was monitored in wild-type or yir035cΔ strains grown in CSM glycerol. The results show that heme synthesis in the yir035cΔ strain is markedly reduced. |
|
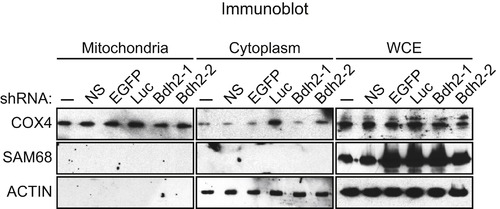
Assessment of the Purity of Mitochondrial Preparations, Related to Figure 6 Immunoblot analysis monitoring the levels of COX4 (a mitochondrial protein), SAM68 (a nuclear protein) and actin (a cytoplasmic protein) in FL5.12 cells treated with one of two unrelated shRNAs targeting Bdh2 or a control enhanced green fluorescent protein (EGFP), luciferase (Luc) or non-silencing (NS) shRNA. The results show that the mitochondrial preparations used in the experiments presented in Figure 6 are free of cytoplasmic and nuclear contaminants. |
|
Additional Experiments, Related to Figure 7 (A) bdh2 is broadly expressed in zebrafish embryos. Whole-mount in situ hybridization analysis reveals that at 28 hr post fertilization (hpf) bdh2 mRNA is widely distributed throughout the zebrafish embryo. As a negative control, in situ hybridization was also performed using a bdh2 sense-strand probe (bottom). (B) Confirmation of decreased hemoglobin levels in Bdh2-depleted zebrafish embryos using a second, unrelated bdh2 MO. (Top) Phenotypic analysis of control MO- and bdh2 MO-injected embryos at 72 hpf. (Bottom) Whole-mount o-dianisidine staining of control MO and bdh2 MO-injected embryos at 72 hpf. Shown are lateral (top) and ventral (bottom) views of the anterior region of embryos. EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Cell, 141(6), Devireddy, L.R., Hart, D.O., Goetz, D.H., and Green, M.R., A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production, 1006-1017, Copyright (2010) with permission from Elsevier. Full text @ Cell