- Title
-
Loss of Smyhc1 or Hsp90alpha1 function results in different effects on myofibril organization in skeletal muscles of zebrafish embryos
- Authors
- Codina, M., Li, J., Gutiérrez, J., Kao, J.P., and Du, S.J.
- Source
- Full text @ PLoS One
|
Knockdown of smyhc1 expression by smyhc1 ATG-MO. A. Western blot analysis shows the effect of smyhc1-MO on the expression of the myosin heavy chain in slow muscles (F59) at 24 and 48 hpf. Anti-γ-tubulin was used as a loading control. B, C. Anti-MyHC antibody (F59) staining shows myosin expression in trunk slow muscles of control (B), or smyhc1 knockdown (C) embryos at 48 hpf. Myosin expression was significantly knocked down in slow muscles. However, myosin expression could be detected in myofibers in the dorsal and myoseptum region of the myotome (arrows) that express smyhc2 and smyhc3. D, E. F59 antibody staining on cross-sections shows MyHC expression in slow muscles (arrows) of control (D) or smyhc1-ATG-MO injected embryos (E) at 48 hpf. F, G. MF20 antibody staining shows MyHC expression in fast muscles of control (F) or smyhc1-ATG-MO (G) injected embryos at 48 hpf. Scale bars = 25 μm in B; 75 μm in D and F. |
|
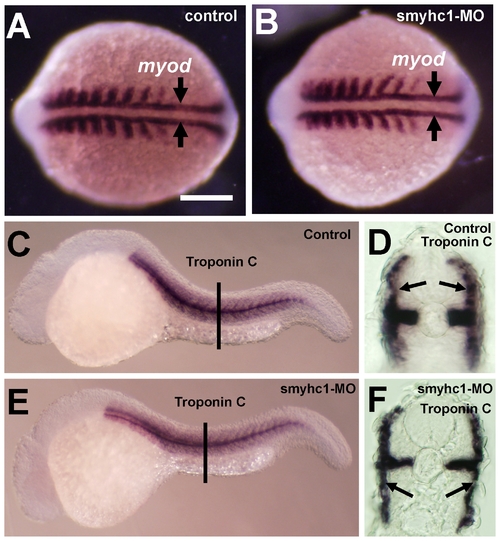
Effects of smyhc1 knockdown on muscle development in zebrafish embryos. A and B. In situ hybridization shows myod expression in control (A) or smyhc1-ATG-MO (B) injected embryos at 14 hpf. Adaxial cells that give rise to slow muscles are indicated by arrows. C–F. In situ hybridization shows slow-specific troponin C expression in control (C, D) or smyhc1-ATG-MO (E, F) injected embryos at 24 hpf. D and F are cross sections of C and E, respectively. Arrows in D and F indicate slow muscles. EXPRESSION / LABELING:
|
|
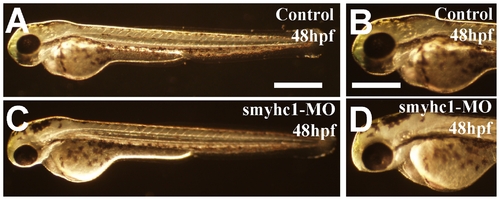
Morphology of control and smyhc1 knockdown embryos at 48 hpf. Morphological comparison of control (C, D) or smyhc1-ATG-MO injected (E, F) embryos at 48 hpf. Scale bars = 30μm in A, 100 μm in C and D. PHENOTYPE:
|
|
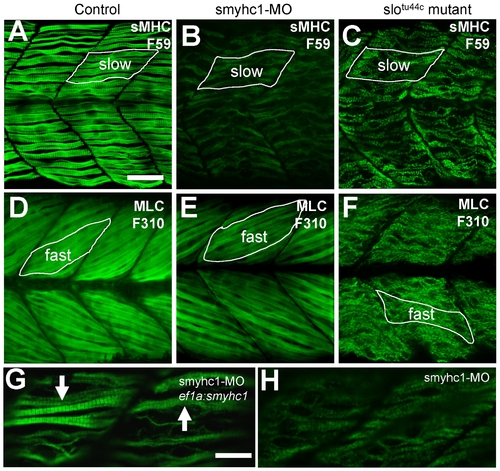
Effects of smyhc1 knockdown or hsp90α1 mutation on myosin thick filament organization in skeletal muscles of zebrafish embryos. A–C. Anti-MyHC antibody (F59) staining shows the organization of thick filaments in trunk slow muscles of control (A), smyhc1 knockdown (B), or slotu44c mutant (C) embryos at 48 hpf. D–F. Anti-MLC antibody (F310) staining shows the organization of thick filaments in trunk fast muscles of control (D), smyhc1 knockdown (E), or slotu44c mutant (F) embryos at 72 hpf. Note, fast fibers project with a 30 degree angle with respect to the axial structure, whereas slow fibers project in parallel to the axial structure. G, H. Anti-MyHC antibody (F59) staining shows the rescue of thick filaments in smyhc1 knockdown zebrafish embryos co-injected with ef1a:smyhc1 DNA construct (G), or ATG-MO alone (H). Scale bar = 25 μm in A, 10 μm in G. EXPRESSION / LABELING:
PHENOTYPE:
|
|
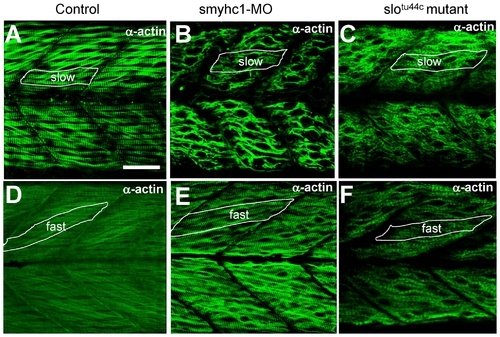
Knockdown of smyhc1 expression or hsp90α1 mutation resulted in defective thin filament organization in skeletal muscles of zebrafish embryos. A–C. Anti-α-actin antibody staining shows the organization of thin filaments in slow muscles of control (A), smyhc1 knockdown (B), or slotu44c mutant (C) embryos at 48 hpf. D, F. Anti-α-actin antibody staining shows the organization of thin filaments in fast muscles of control (D), smyhc1 knockdown (E), or slotu44c mutant (F) embryos at 72 hpf. Scale bar = 25 μm in A. EXPRESSION / LABELING:
PHENOTYPE:
|
|
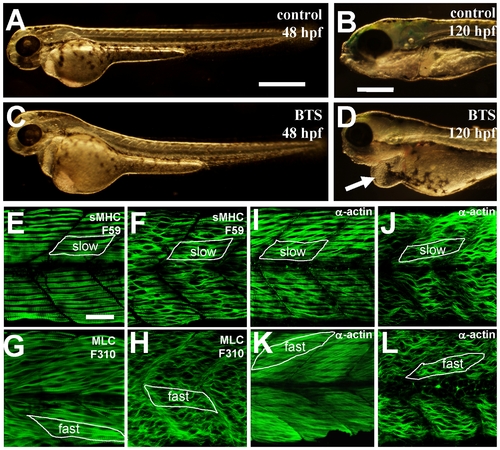
BTS inhibits skeletal muscle contraction and suppresses thick and thin filament assembly in skeletal muscles of zebrafish embryos. A–D. Morphological comparison of control (A, B) or BTS-treated (C, D) embryos at 48 hpf (A, C) and 120 hpf (B, D). Compared with control (B), BTS-treated embryos (D) showed a clear edema (indicated by the arrow) at 120 hpf. E and F. Anti-MyHC antibody (F59) staining shows the organization of thick filaments in slow muscles of control (E) or BTS-treated (F) embryos at 60 hpf. G and H. Anti-MLC antibody (F310) staining shows the organization of thick filaments in fast muscles of control (G) or BTS-treated (H) embryos at 72 hpf. I and J. Anti-α-actin antibody staining shows the organization of thin filaments in slow muscles of control (I) or BTS-treated (J) embryos at 60 hpf. K and L. Anti-α-actin antibody staining shows the organization of thin filaments in fast muscles of control (K) or BTS-treated (L) embryos at 72 hpf. Scale bars = 100 μm in A and B, 25 μm in E. |
|
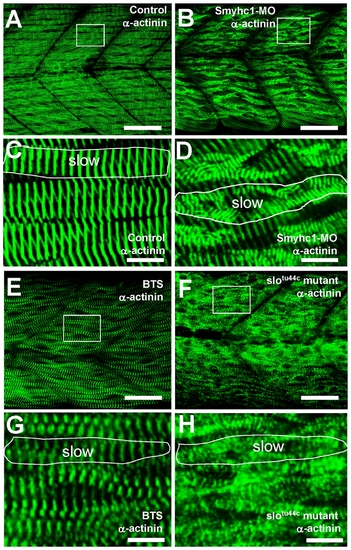
The effect of smyhc1 knockdown, BTS treatment or hsp90α1 mutation on Z body formation, and Z-line organization in skeletal muscles of zebrafish embryos. Anti-α-actinin antibody staining shows the Z-disk organization in control (A, C), smyhc1 knockdown (B, D), BTS-treated (E, G), or slotu44c mutant (F, H) embryos at 60 hpf. C, D, G and H are high magnifications of A (control), B (smyhc1 knockdown), E (BTS treated) and F (slotu44c mutant), respectively. Scale bar = 25 μm in A, B; 20 μm in E, F; 4 μm in C, D, G, H. |
|
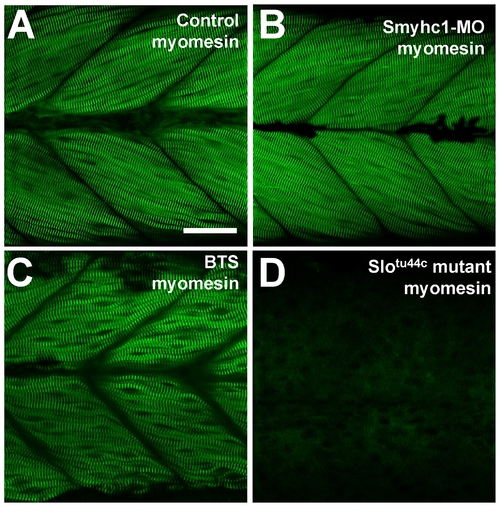
The effect of smyhc1 knockdown, BTS treatment or hsp90α1 mutation on M-line organization in skeletal muscles of zebrafish embryos. Anti-myomesin antibody staining shows the M-line organization in control (A), smyhc1 knockdown (B), BTS-treated (C), or slotu44c mutant (D) embryos at 72 hpf. Scale bar = 25 μm in A. EXPRESSION / LABELING:
PHENOTYPE:
|