- Title
-
The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication
- Authors
- Yabe, T., Ge, X., and Pelegri, F.
- Source
- Full text @ Dev. Biol.
|
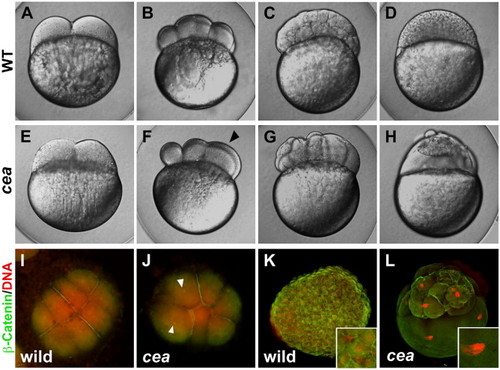
Phenotype of maternally mutant cea embryos. (A–H) Side views of live wild-type (A–D) and mutant (E–H) embryos at the following time points: (A, E) 45 min post fertilization (p.f.), (B, F) 1.25 h p.f., (C, G) 2.25 h p.f., (D, H) 3 h p.f. (corresponding, in wild-type, to the 2-, 8-, 128- and 1000-cell stages, respectively). Maternally mutant cea embryos show a normal first cell division (E) but fail in a fraction of subsequent divisions (F, arrowhead), leading to blastula with irregularly sized blastomeres (G) and largely syncytial embryos (H). (I–L) Animal views of fixed wild-type (I, K) and mutant (J, L) embryos labeled to detect β-catenin (green) and DNA (red). At 1.25 h p.f. (8-cell stage in wild-type; panels I, J), the mutant embryo reveals a failure of DNA and cell division in a fraction of the blastomeres (arrowheads indicate nuclei of cells that did not undergo division). At 3 h p.f. (1000-cell stage in wild-type; panels K, L), mutant embryos exhibit enlarged nuclei (inset in panel L, compare to panel K). |
|
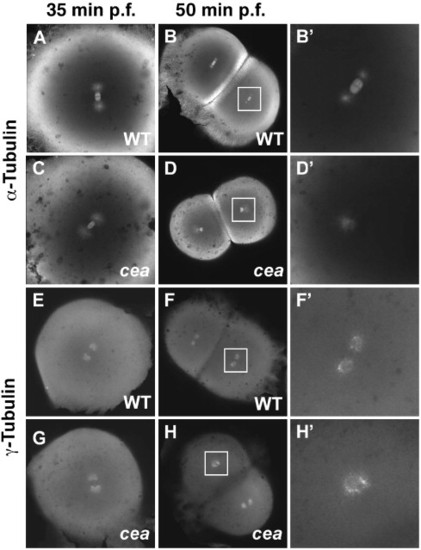
Spindle organization and centrosome duplication defects in maternally mutant cea embryos. (A–D′) Fixed wild-type (A, B′) and mutant (C, D′) embryos labeled with anti-α-tubulin antibody. Spindle organization is normal in the mutant immediately prior to the first cell division (35 min p.f.; panel C; compare to panel A), but is defective in a fraction of blastomeres in the next cleavage cycle (50 min p.f.; panels D, D′; compare to panels B, B′). (E–H′) Fixed wild-type (E, F′) and mutant (G, H′) embryos labeled with anti-γ-tubulin antibody. Centrosome duplication appears normal immediately prior to the first cleavage division (35 min p.f.; (G), compare to (E)) but is defective in a fraction of the blastomeres in the following cleavage cycle (50 min p.f.; panels H, H2, compare to panels F, F′). Animal views. Panels B′, D′, F′, H′ are enlargements of the area indicated by the squares in panels B, D, F, H, respectively. PHENOTYPE:
|
|
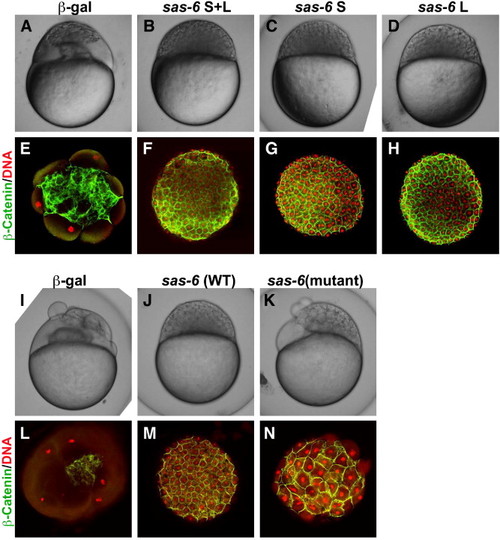
Genetic rescue of the maternal cea mutant phenotype by sas-6 expression. Side views of live embryos at the 1000-cell stage (A–D, I–K) and animal views of fixed embryos labeled to detect β-catenin and DNA (E–H, L–N). Injection of mRNA coding for Sas-6L and Sas-6S results in a similar extent of rescue (panels B–D, F–H compare to panels A, E). Injection of mRNA coding for the mutated cea protein results in significant rescue (panels K, N; compare to panels I, L), which is nevertheless not as effective as the rescue conferred by wild-type mRNA injected in sibling embryos (J, M). |
|
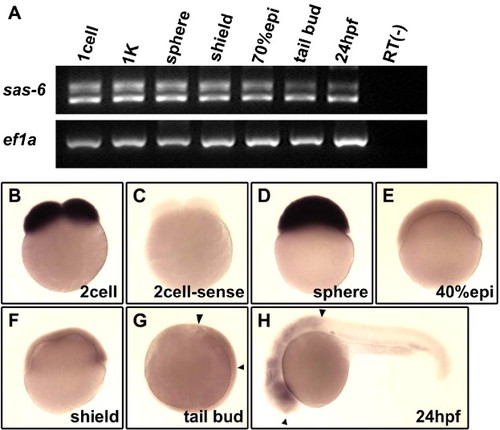
Expression of zebrafish cea/sas-6 during early embryogenesis. (A) RT–PCR analysis shows that both splice forms are expressed maternally and are present during early embryogenesis. (B–H) In situ hybridization analysis shows the presence of maternal transcripts (panel B, compared to control sense probe in panel C), which remain until the sphere stage (D), and subsequently a lower level of ubiquitous expression during gastrulation (E, F) and enrichments in the brain region (G, H; flanked by arrowheads). |
|
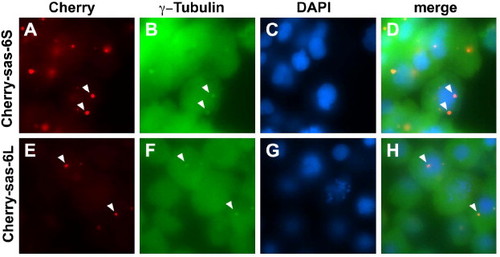
Cea/Sas-6 localizes to centrosomes. Expressed fusions of mCherry and Sas-6 splice forms colocalize in cytoplasmic structures containing γ-tubulin (arrowheads), a marker for centrosomes. Fields of cells in embryos fixed at 50% epiboly. |
|
One-cycle cell division delay in embryos from mutant cea fathers. Live embryos at the indicated time points. (A–F) are side views, (B′, D′) are animal views of the same embryos shown in panels B, D. A fraction of paternally mutant cea embryos show a one-cycle delay in cell division (panel C, compare to panel A), but subsequently resume normal cleavage (D, D′). At 5 days p.f., embryos that undergo a one-cycle delay in cell division (F) exhibit heart edema (asterisk) and lack swim-bladder inflation (arrow). (G, H) Metaphase spreads from 24-h embryos. Wild-type embryos contain a normal complement of 50 chromosomes (G), while paternally mutant cea embryos that experience a one-cell cycle delay in the first cell division contain a duplicated number of chromosomes (H). |
|
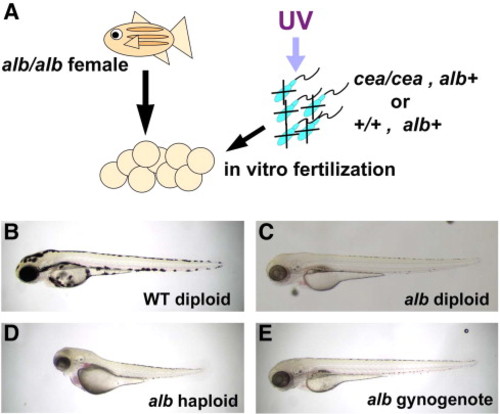
Induction of gynogenesis by sperm from cea mutant males. (A) Diagram of gynogenesis procedure. (B–E) Side views of 3-day p.f. embryos. For comparison, panels B and C show, respectively, control diploid wild-type and albino pigmented derived from standard natural crosses. (D) albino haploid embryo derived from fertilization of albino females with UV-inactivated wild-type sperm. (E) A fraction of embryos fertilized with UV-inactivated sperm from cea mutant males develops into albino diploid gynogenotes. |
|
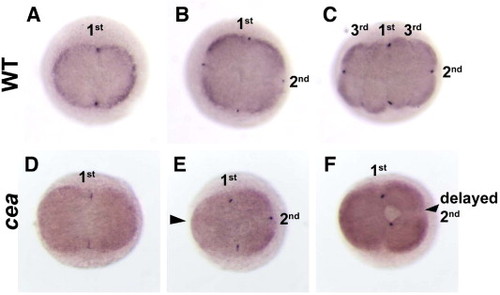
Germ plasm recruitment fails after a one-cycle delay and cleavage resumption. In situ hybridization to label vasa mRNA in wild-type (A–C) and maternally mutant cea embryos (D–F) at the 45 min p.f. (2-cell in wild-type; A, D), 1 h p.f. (4-cell in wild-type; B, E) and 1.25 h p.f. (8-cell in wild-type; C, F). In wild-type embryos, germ plasm accumulates at the furrows of the first and second cell divisions (A, B), but not for the third cell division (C). In mutant embryos, germ plasm accumulates normally in the furrow of the first cell division (D), but fails to accumulate in both furrows that do not form in the second cell cycle (E, arrowhead) and furrows that form after a one-cycle delay of the second cleavage division (F, arrowhead). 1st, 2nd and 3rd indicate the cleavage furrows of the first, second and third cleavage cycle. |
Reprinted from Developmental Biology, 312(1), Yabe, T., Ge, X., and Pelegri, F., The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication, 44-60, Copyright (2007) with permission from Elsevier. Full text @ Dev. Biol.