- Title
-
Retinoic acid signaling restricts the size of the first heart field within the anterior lateral plate mesoderm
- Authors
- Duong, T.B., Holowiecki, A., Waxman, J.S.
- Source
- Full text @ Dev. Biol.
|
RA signaling promotes specification of posterior nkx2.5+ progenitors. (A–F) ISH for nkx2.5:Kaede (Kaede) in DMSO-treated (control) and DEAB-treated embryos at the 14s, 18s, and 20s stages (16, 18, and 19 hpf). Brackets in A and B indicate the length and width of the nkx2.5:Kaede expression. Nkx2.5 expression is expanded laterally and posteriorly and the posterior pharyngeal artery progenitors are not a distinct aggregate in RA deficient embryos. Views are dorsal with anterior up. Arrows in C and E indicate the space between the anterior nkx2.5+ and posterior nkx2.5+ aggregates. Arrows in D and F indicate the posterior limit of nkx2.5 expression and lack of posterior nkx2.5 aggregates. n = 25 for all conditions examined. |
|
Cardiac progenitors are expanded posteriorly within the nkx2.5+ progenitor field. (A,B,D,E,G,H,J,K) Photoconversion-mediated lineage tracing of nkx2.5:Kaede+ progenitor cells in DMSO- and DEAB-treated embryos at the 20s stage (19 hpf) and at 48 hpf. Views in A,D,G, J are dorso-lateral with anterior up. Views in B,E,H,K are lateral with anterior right and dorsal up. a – anterior, p – posterior, c - cardiac cone. Embryos at 48 hpf (B,E,H,K) are the same embryos shown with photoconverted cells at the 20s stage (19 hpf) in (A,D,G,J). Arrow in B indicates photoconverted cells in the ventral aorta and OFT. (C,F,I,L) Schematics summarizing the lineage tracing in the anterior and posterior lateral nkx2.5:Kaede+ fields. V – ventricle, A – Atrium. In the anterior lateral nkx2.5+ region, 4 of 4 control embryos had labeled cells give rise to the ventral aorta/OFT, while 1 of the 4 also labeled the 3rd PAA. 4 of 4 DEAB-treated embryos with nkx2.5:Kaede+ cell clusters labeled in similar regions gave rise to the ventral aorta, OFT, and CMs. In the posterior lateral nkx2.5:Kaede+ field, 6 of 6 control embryos had photoconverted cells contribute to the 3rd PAA. 8 of 8 DEAB-treated embryos had photoconverted cells give rise to the ventral aorta, OFT, and CMs. Arrow in H indicates photoconverted cells in the 3rd PAA. Arrowheads in E and K indicated photoconverted CMs at the arterial pole. Scale bars – 100 μm. |
|
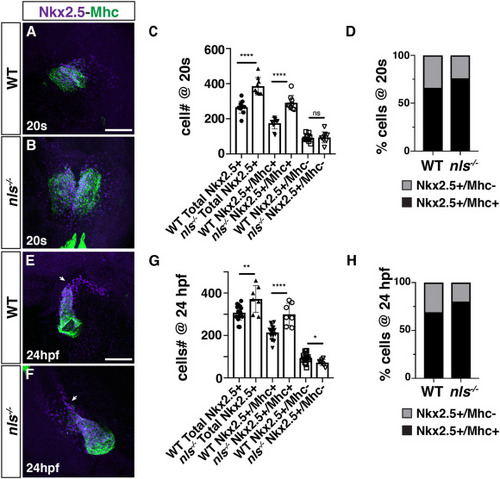
RA restricts the size of the FHF and maintains the SHF progenitor population in nls mutant embryos. (A, B) IHC for Nkx2.5 (purple) and Mhc (green) in WT sibling and nls mutant embryos at the 20s stage (19 hpf). Nls mutant hearts were sometimes delayed in forming the cardiac cone relative to WT siblings. Views are dorsal with anterior up. Scale bar – 100 μm. (C) Quantification of the number of Nkx2.5+/Mhc+ and Nkx2.5+/Mhc-cells in WT sibling and nls mutant embryos at the 20s stage (19 hpf). ∗∗∗∗ indicates p < 0.0001. ns – not significant. (D) Percentage of Nkx2.5+/Mhc+ and Nkx2.5+/Mhc-cells within the hearts of WT sibling and nls mutant embryos at the 20s stage (19 hpf). (E, F) IHC for Nkx2.5 (purple) and Mhc (green) in WT sibling and nls mutant embryos at 24 hpf. Frontal views with the arterial pole up. Arrows indicate the border between Nkx2.5+/Mhc+ and Nkx2.5+/Mhc-cells. Scale bar – 100 μm. (G) Quantification of the number of Nkx2.5+/Mhc+ and Nkx2.5+/Mhc-cells in WT sibling and nls mutant embryos at 24 hpf. ∗ indicates p = 0.0253, ∗∗ indicates p = 0.0026, ∗∗∗∗ indicates p < 0.0001. (H) Percentage of Nkx2.5+/Mhc+ and Nkx2.5+/Mhc-cells within the hearts of WT sibling and nls mutant embryos at 24 hpf. |
|
RA restricts the size of the FHF and maintains the SHF progenitor population in DEAB-treated embryos. (A, B) IHC for Nkx2.5 (purple) and GFP (myl7:GFP - green) in DMSO- and DEAB-treated embryos at the 20s stage (19 hpf). The cardiac cone was sometimes delayed in forming in DEAB-treated embryos relative to DMSO-treated control embryos. Views are dorsal with anterior up. Scale bar – 100 μm. (C) Quantification of the number of Nkx2.5+/GFP+ and Nkx2.5+/GFP- cells in DMSO- and DEAB-treated embryos at the 20s stage (19 hpf). ∗∗∗ indicates p ≤ 0.0004. (D) Percentage of Nkx2.5+/GFP+ and Nkx2.5+/GFP- cells within the hearts of DMSO- and DEAB-treated embryos at the 20s stage (19 hpf). (E, F) IHC for Nkx2.5 (purple) and GFP (myl7:GFP - green) in DMSO- and DEAB-treated embryos at 24 hpf. Frontal views with the arterial pole up. Arrows indicate the border between Nkx2.5+/GFP+ and Nkx2.5+/GFP- cells. Scale bar – 100 μm. (G) Quantification of the number of Nkx2.5+/GFP+ and Nkx2.5+/GFP- cells in DMSO- and DEAB-treated embryos at 24 hpf. ∗∗ indicates p ≤ 0.0060, ∗∗∗∗ indicates p < 0.0001. (H) Percentage of Nkx2.5+/GFP+ and Nkx2.5+/GFP- cells within the hearts of DMSO- and DEAB-treated embryos at 24 hpf. |
|
Reduced ventricular CMs differentiate at the arterial pole of RA-deficient embryos. (A-B″) DMSO- and DEAB-treated myl7:nls-KikGR embryos at 48 hpf. Embryos were photoconverted at 24 hpf. Dashed line demarcates the border between green-only and green+/red + ventricular CMs. Views are lateral with the arterial pole up. Scale bars – 100 μm. (C) Quantification of the number of green-only cells within the ventricle. ∗∗∗∗ indicates p < 0.0001. |
|
SHF markers of the arterial pole are reduced in RA-deficient embryos. (A,B) ISH for mef2cb at 30 hpf in DMSO- and DEAB-treated embryos. (C,D) ISH for ltbp3 at 30 hpf in DMSO- and DEAB-treated embryos. Number of embryos examined per condition: mef2cb DMSO-treated n = 31; DEAB-treated n = 18; for ltbp3 DMSO-treated n = 18; DEAB-treated n = 16. Views are dorsal with anterior up. Arrows indicate the length of expression at the arterial poles. |
|
Smooth muscle within the OFT is absent in RA-deficient embryos. (A–D) IHC of Elnb (green) and Mhc (red) in hearts of (A,B) WT sibling and nls mutant embryos and (C,D) DMSO- and DEAB-treated embryos at 72 hpf. Number of embryos examined per condition: WT n = 6, nls n = 8, DMSO-treated n = 12, DEAB-treated n = 9. Views are lateral with arterial pole of the heart up. Arrows indicate the Elnb in WT sibling and DMSO-treated and the lack of Elnb in nls mutant and DEAB-treated at the arterial poles of the hearts. Scale bar – 50 μm. |
|
Pacemaker CMs do not differentiate in RA-deficient embryos. (A,B) IHC of Isl1 (green), Mhc (red), Amhc (blue) in hearts of DMSO- and DEAB-treated embryos at 48 hpf. Number of embryos examined: DMSO-treated n = 25, DEAB-treated n = 21. Arrows in A and B indicate Isl1 expression and the lack of Isl1 at the venous poles of the hearts in control and DEAB-treated embryos, respectively. (C,D) IHC of GFP (green), Mhc (red), Amhc (blue) in hearts of DMSO- and DEAB-treated sqet33-mi59B embryos at 48 hpf. Number of embryos examined: DMSO-treated n = 11, DEAB-treated n = 11. Arrows in C and D indicate the sqet33-mi59B GFP expression and its reduced expression at the venous poles of the hearts in control and DEAB-treated embryos, respectively. The remaining sqet33-mi59B expression in DEAB-treated embryos is adjacent to the atrial CMs. Views are ventral with anterior up. Scale bar – 50 μm. |
|
Anterior pharyngeal muscles are unaffected in RA-deficient embryos. (A-B″) IHC for ZsYellow+ (green) and Mhc+ (red) pharyngeal muscles in DMSO- and DEAB-treated nkx2.5:ZsYellow embryos at 72 hpf. Views are frontal with anterior left and dorsal up. Brackets indicate the dorsal pharyngeal muscles. Dorsal mandibular arch: lap - levator arcus palatini, do - dilatator opercula. Dorsal hyoid arch: ah - adductor hyomandibulae, ao - adductor opercula. The dashed lines indicate the nkx2.5:ZsYellow+ muscles. The dorsal mandibular and hyoid arch muscles are dysmorphic in RA-deficient embryos. Number of embryos examined: DMSO-treated n = 9, DEAB-treated n = 6. Scale bar – 33 μm. |
|
Neural crest-derived CMs are not affected in RA-deficient embryos. (A,B) IHC for mCherry (red) and Mhc (green) in DMSO- and DEAB-treated NC:mCherry embryos at 72 hpf. Views are ventral with anterior up. Arrows indicate labeled CMs in hearts. (C) Quantification of the NC:mCherry+ cells in the hearts of DMSO-treated and DEAB-treated embryos. Scale bar – 100 μm. |
|
Model of early RA requirements in patterning the zebrafish heart. RA signaling restricts the cardiac progenitor populations within the ALPM. Normally, FHF (green) and anterior SHF (purple) lie adjacent to each other within bilateral populations. Posterior RA signaling limits the boundary of the cardiac progenitor field. As the tube forms, SHF-derived cells are added to the arterial pole of the heart tube, giving rise to ventricular CMs and smooth muscle in the bulbus arteriosus (yellow). At the venous pole, pacemaker CMs (blue) differentiate. In the absence of RA, the FHF progenitors are expanded, while the number of SHF progenitors are actually not initially affected, they may be expanded posteriorly within the ALPM. However, the SHF progenitors that will give rise to the OFT prematurely differentiate, leading to a loss of the SHF-derived-arterial pole and OFT in the enlarged hearts. Pacemaker CMs fail to differentiate at the venous pole of the enlarged hearts in RA-deficient embryos. |
Reprinted from Developmental Biology, 473, Duong, T.B., Holowiecki, A., Waxman, J.S., Retinoic acid signaling restricts the size of the first heart field within the anterior lateral plate mesoderm, 119-129, Copyright (2021) with permission from Elsevier. Full text @ Dev. Biol.