- Title
-
Orientation-Selective Retinal Circuits in Vertebrates
- Authors
- Antinucci, P., Hindges, R.
- Source
- Full text @ Front. Neural Circuits
|
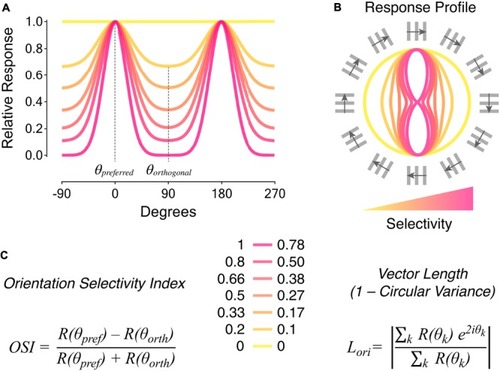
Metrics to quantify orientation selectivity in neural responses. |
|
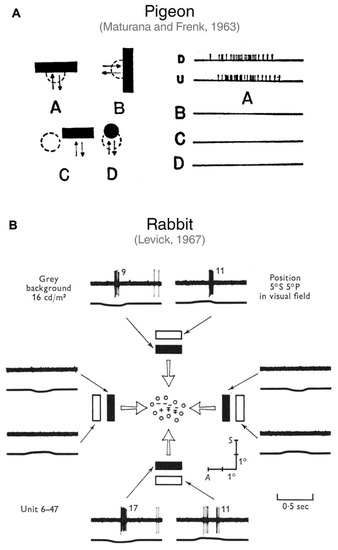
First studies describing orientation-selective ganglion cells in vertebrate retinae. |
|
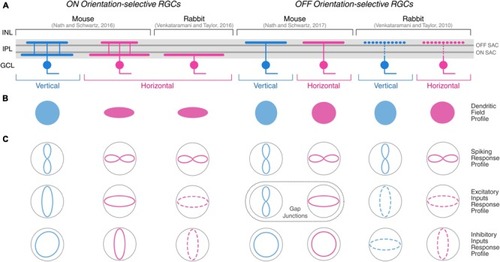
Morphological and physiological features of orientation-selective retinal ganglion cells in mouse and rabbit. Schematic summarizing the morphological |
|
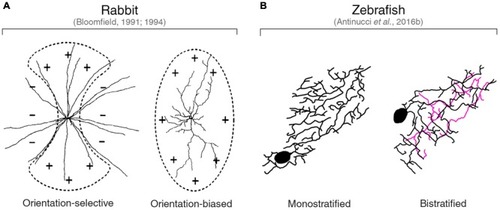
Orientation-tuned amacrine cells in rabbit and zebrafish. |
|
Working models of orientation-selective retinal circuits in vertebrate retinae. |