- Title
-
The epigenetic regulator Histone Deacetylase 1 promotes transcription of a core neurogenic programme in zebrafish embryos
- Authors
- Harrison, M.R., Georgiou, A.S., Spaink, H.P., and Cunliffe, V.T.
- Source
- Full text @ BMC Genomics
|
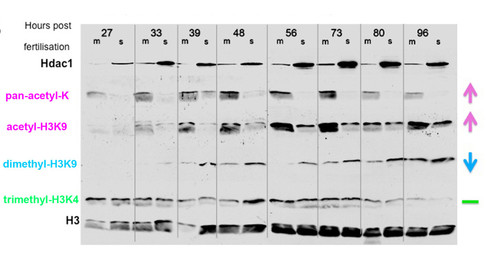
Hdac1 inhibits Histone acetylation and promotes H3K9 methylation levels in the zebrafish embryo. Levels of Hdac1 protein and covalently modified histones in hdac1 mutant (m) and wild-type (s) embryos aged 27, 33, 39, 48, 56, 73, 80 and 96 hpf, visualised by Western blot analysis using anti-Hdac1 and histone modification-specific antibodies. Hdac1 protein visualised with an anti-Hdac1 antibody is shown on the top row. Antibodies specific for the following histone modifications were: Pan-acetyl lysine; acetyl-H3K9; dimethyl-H3K9, trimethyl-H3K4. An anti-H3 antibody was used as a loading control (bottom row). |
|
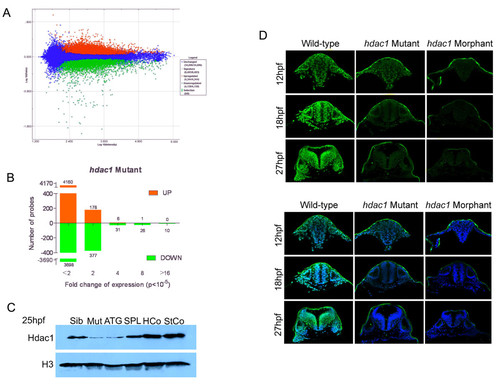
Phenotypic analysis of Hdac1-deficient embryos. (A) Scatter plot of probe intensities from hdac1 mutant versus sibling comparisons. Up-regulated and down-regulated probes in hdac1 mutants are coloured red or green, respectively. The averaged ratio values are plotted on the y-axis and the averaged expression values are plotted on the x-axis, both of which are expressed in a log10 scale. The threshold for statistically significant differential expression was set at p < 10-5. (B) Analysis of the fold-change distribution of Hdac1-regulated probes identified in (A). (C) Microinjection of Hdac1 morpholinos reduces Hdac1 protein abundance in zebrafish embryos. Western blotting for Hdac1 protein in hdac1 mutant (Mut), wild-type (Sib), Hdac1ATG1 morphant (ATG), Hdac1SPL1 morphant (SPL), Hdac1Control morphant (HCo) and Standard Control morphant (StCo) embryos. (D) Reduced immunostaining for Hdac1 in hdac1 mutant and Hdac1ATG1 morphant embryos. Transverse confocal sections through the hindbrain of 12 hpf, 18 hpf and 27 hpf wild-type, hdac1 mutant and Hdac1ATG1 morphant embryos immunostained for Hdac1 protein (green in upper and lower panels) and counterstained with DAPI (blue, in lower panel only). |
|
Expression of the Hdac1 neurogenic programme is stably attenuated in Hdac1ATG1 morphant embryos. (A) 6-somite stage (12 hpf) embryos and (B) 27 hpf embryos injected with standard Control + p53 morpholinos or Hdac1ATG1 + p53 morpholinos, analysed for expression of neurod4, ascl1b, neurod, dlb, lhx9 and sox2. Expression of neurod4, ascl1b, neurod, dlb and lhx9 was reduced in Hdac1-deficient embryos. In contrast, the expression pattern of sox2, which did not exhibit a statistically significant change in transcript abundance in the microarray analysis, was unaltered in 12 hpf Hdac1ATG1 morphant embryos. |
|
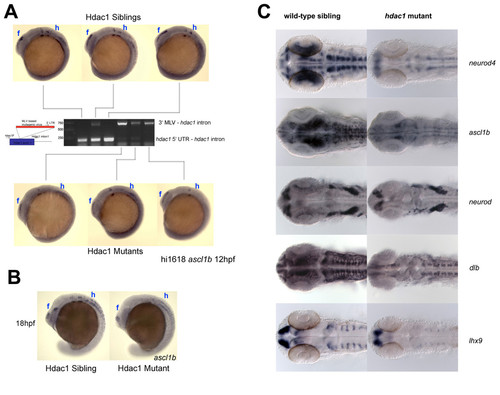
Expression of the Hdac1 neurogenic programme is stably attenuated in hdac1 mutant embryos. (A) 12 hpf embryos were sorted according to ascl1b expression level and individuals were then genotyped by PCR. All embryos with strong ascl1b expression had the wild type hdac1 allele (lower band) and were therefore wild-type siblings, whereas embryos with a weak ascl1b expression pattern carried only the mutant hdac1 allele (upper band) and were therefore hdac1 mutants. (B) Similar results to those shown in (A) were obtained for 18 hpf embryos. In both (A) and (B), the forebrain (f) and hindbrain (h) regions of Hdac1-regulated ascl1b expression are indicated. (C) Expression of the Hdac1 neurogenic programme is attenuated in 27 hpf hdac1 mutant embryos. Expression of neurod4, ascl1b, neurod, dlb and lhx9 in 27 hpf hdac1mutant and wild-type sibling embryos. Expression of each of these genes is reduced in the CNS of hdac1 mutant embryos. |
|
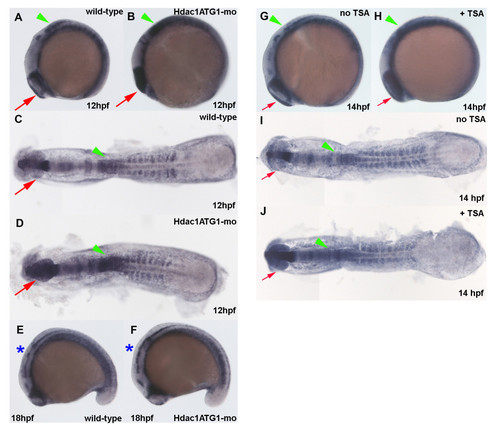
Increased her6 expression in the CNS of Hdac1ATG1 morphants and TSA-treated embryos during early neurogenesis. (A-F) Wild-type sibling (A, C, E) and Hdac1ATG1 morphant (B, D, F) embryos were analysed for expression of her6 at 12 hpf (A, B, C, D) and 18 hpf (E, F). (G-J) Embryos were cultured in the absence (G, I) or presence (H, J) of the HDAC inhibitor TSA (1 μM) from 10-14 hpf, then analysed for expression of her6. In dorsal and lateral views, anterior is to the left. Green arrowheads indicate caudal hindbrain, which exhibits increased her6 expression in Hdac1ATG1 morphants at 12 hpf and in TSA-treated embryos at 14 hpf; red arrows indicate optic vesicles, which exhibit increased her6 expression in Hdac1ATG1 morphants at 12 hpf and in TSA-treated embryos at 14 hpf; blue asterisks indicate hindbrain, which exhibits increased increased her6 expression in Hdac1ATG1 morphant embryos at 18 hpf. |
|
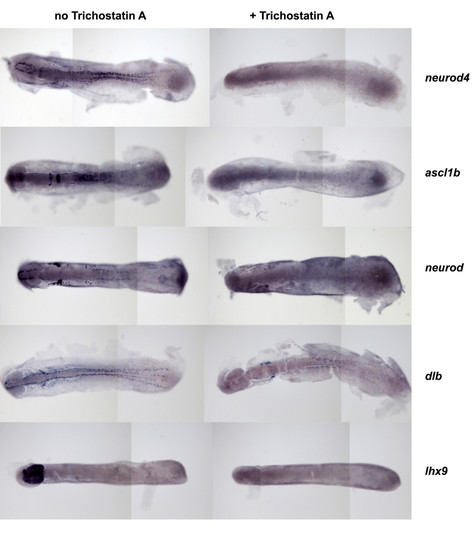
Trichostatin A (TSA) extinguishes neurod4, ascl1b, neurod, dlb and lhx9 expression during early neurogenesis. Embryos were cultured in the absence or presence of TSA (1 μM) from 10-14 hpf, then analysed for expression of genes as indicated. In all panels, anterior is to the left. |
|
Efficient immunoprecipitation of Hdac1 protein from embryonic chromatin by anti-Hdac1 antibody. Crosslinked, sonicated chromatin was prepared from 12 hpf zebrafish embryos, then incubated with anti-Hdac1 antibody and negative control IgG. Immune complexes were precipitated with Protein G-agarose and both immunoprecipitated proteins and unbound proteins were analysed by SDS-PAGE and Western blotting with the anti-Hdac1 antibody, with an equivalent sample of input chromatin run alongside. All Hdac1 protein in the input sample was recovered in the anti-Hdac1 immunoprecipitate, whereas none of the Hdac1 protein was immunoprecipitated by the negative control IgG and it remained in the unbound fraction. |