- Title
-
Brain asymmetry is encoded at the level of axon terminal morphology
- Authors
- Bianco, I.H., Carl, M., Russell, C., Clarke, J.D., and Wilson, S.W.
- Source
- Full text @ Neural Dev.
|
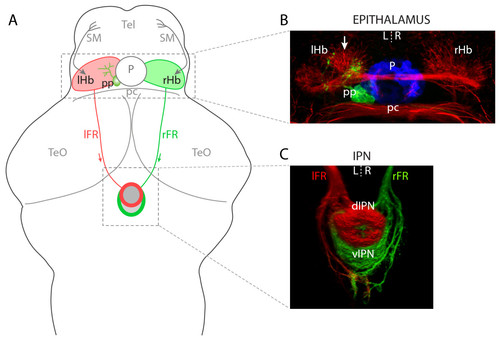
Left-right asymmetries in the telencephalon-habenula-IPN pathway. (a) Schematic of a 4 dpf larval zebrafish brain. The bilaterally paired epithalamic habenular (Hb) nuclei receive afferent inputs from nuclei in the telencephalon (Tel) via the stria medullaris (SM; grey arrows; asymmetric innervation of the R habenula [20] is not indicated) and diencephalon (not shown). Habenular neurons send efferent projections via the fasciculi retroflexus (FR) to the interpeduncular nucleus (IPN) in the ventral midbrain. L- and R-sided habenular axons are segregated along the DV axis of the IPN wherein the L habenula predominantly innervates the dIPN and makes less substantial projections to the vIPN whereas the vast majority of R-sided axons terminate in the vIPN. In addition to the habenulae, the epithalamus contains the pineal complex, comprising the photoreceptive pineal (P) and parapineal (pp) nuclei. The parapineal is located on the L side of the dorsal midline and projects efferent axons that exclusively innervate the L habenula. (b) Neuroanatomical asymmetries in the epithalamus. Anti-acetylated tubulin immunostaining (red) reveals the L habenula displays a greater density of neuropil, especially in the dorso-medial aspect of the nucleus (arrow). The pineal (blue) and parapineal (green) nuclei are visualized by expression of GFP in a Tg(foxD3:GFP) transgenic larva. Parapineal efferent axons predominantly terminate in the asymmetric medial neuropil of the L habenula. (c) Three-dimensional reconstruction of habenular axon terminals in the ventral midbrain, labeled using lipophilic carbocyanine dyes applied to the habenulae. L-sided axons were labeled with DiD (red) and R-sided axons with DiI (green). The dIPN is almost exclusively innervated by L-sided axons, whereas the ventral target receives a majority of R-sided inputs. All panels show dorsal views, anterior top. pc, posterior commissure; TeO, optic tectum. EXPRESSION / LABELING:
|
|
Habenular neurons have a stereotypical unipolar morphology and their axons terminate in spiral-shaped arbors that display multiple midline crossing. (a) Three-dimensional reconstruction showing a R habenular (rHb) projection neuron in an intact 4 dpf larval zebrafish brain. Arrow indicates direction of axonal projection within the FR, from the rHb to the IPN. (b) A single R habenular neuron labeled by focal electroporation and visualized by anti-GFP immunostaining (brown). The image shows the dorsal diencephalon of a dissected brain of a 4 dpf larva. Dotted lines show the borders of the habenulae and the position of the pineal stalk (PS). (c) Single-depth confocal section through the habenulae of a 4 dpf Tg(ET16:GFP) transgenic larva in which a subset of habenular neurons express GFP. In each nucleus the neuronal somata are arranged as ovoid shells surrounding a central neuropil domain (asterisks). It is in this domain that neurons elaborate their dendritic arbors. (d-f) Examples of the somata, dendritic arborizations and proximal axons of habenular neurons labeled by focal electroporation of membrane-tethered GFP. (d, e) Neurons with long processes that give rise to a dendritic tree and axon. (f) Two neurons with intertwined dendritic arbors close to the cell body. In (d-f) an arrowhead marks the proximal axon, and the laterality (left (L) or right (R)) of the habenular neuron(s) is indicated bottom right. (g) Confocal z-projection within the IPN showing a single axonal arbor elaborated by a R habenular projection neuron labeled by focal electroporation. The arbor crosses the ventral midline (dotted line) multiple times. Branches can also reverse direction such that they encircle the IPN in opposite senses (green arrows show examples). (h) High-magnification images of a section of a habenular axon arbor crossing the ventral midline (dotted line). The neuron was electroporated with a construct driving expression of cytosolic DsRed (red, middle panel) and a Syp-GFP fusion protein (green, lower panel). Syp-GFP puncta are present on both sides of the midline and co-localise with axonal variscocities. Scale bars: (a) 100 μm; (c) 50 μm; (e, h) 10 μm; (g) 20 μm. |
|
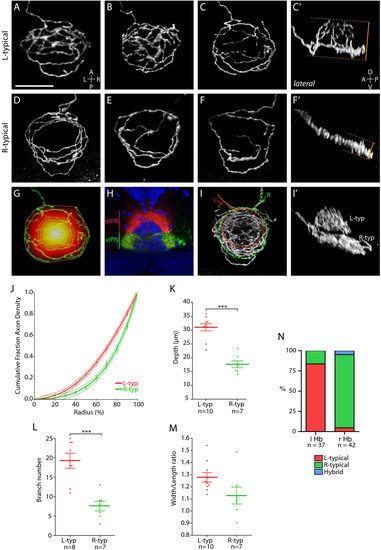
Two distinct axon arbor morphologies formed by habenular projection neurons. (a-g, i) Dorsal, (c′, f′, i′) lateral and (h) transverse confocal images of the IPN region in 4 dpf larvae. In all panels habenular axons were labeled by electroporation except for (h) where lipophilic dye tracing was used to visualize axon terminals. Panels show three-dimensional reconstructions except (h), which is a single-depth confocal image. (a-c′) Three examples of L-typical (L-typ) arbors. These arbors are shaped like a domed crown and arborize over a considerable DV extent (compare dorsal (c) and lateral (c′) views of an example L-typical arbor). See also Additional file 2. (d-f′) Examples of R-typical (R-typ) arbors, which are considerably flatter. See also Additional file 3. Panel (d) shows two R-typical arbors formed by two R habenular neurons. In (c′, f′) orange dotted lines and bars indicate how DV extent was measured for L-typical and R-typical arbors (see Materials and methods). (g) Schematic to illustrate how the radial distribution of axon density was measured. The perimeter of the arbor (indicated by white line) was defined using the convex hull method. The area covered by the arbor was then divided into ten equally spaced concentric shells (colored white-red) centered on the centroid of the hull. After thresholding, the number of pixels representing axon branches was then quantified for each shell (see Materials and methods). (h) Transverse confocal section through the IPN showing the entire contingent of L and R habenular axon terminals labeled using DiD (red) and DiI (green), respectively, in a Tg(h2afz-GFP) transgenic larva to label the nuclei of IPN neurons (blue). Most L-sided neurons innervate the dIPN whereas the majority of R-sided axons terminate in the vIPN. (i, i′) L- and R-sided axons labeled in the same larva. L-typical arbors are located dorsal to R-typical arbors. (j) Cumulative fraction of axon density plotted against radius (measured from centroid of the convex hull to the perimeter) for ten L-typical and seven R-typical arbors. The data are fit by fourth-order polynomial models (solid lines). Dotted lines indicate the 95% confidence band of each best-fit curve. R-typical arbors have a greater proportion of axon density localized towards the perimeter of the arbor. (k) L-typical arbors extend over a greater DV extent than R-typical arbors. (l) L-typical arbors have more branch points than R-typical arbors. (m) The width/length ratio of L-typical arbors shows a trend towards higher values than for R-typical arbors. (n) The majority of L-sided habenular neurons elaborate L-typical arbors whereas most R-sided neurons form R-typical axon arbors. Horizontal lines indicate mean values and error bars show standard error of the mean. ***p < 0.001. |
|
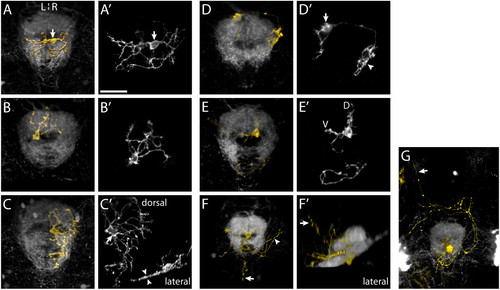
Morphology of IPN neurons. (a-g, f′) Three-dimensional projections of confocal z-stacks showing electroporated IPN neurons expressing membrane-Cherry (gold) and the surrounding IPN neuropil (grey), visualized using the Tg(ET16:GFP) transgenic line. All panels are dorsal views, anterior top, except (f′), which is a lateral view, anterior right. (a′-e′) Maximum intensity projections of deconvolved confocal z-stacks reveal the detailed morphology of IPN neurons. (a, a′) An interneuron in the vIPN that extends neurites exclusively within the vIPN neuropil. The soma (arrow) is located on the R of the midline but the neurites enter the neuropil on both L and R sides. A second, more weakly labeled neuron is also visible. (b, b′) The soma of this neuron, as with most IPN neurons, occupies the central cellular ′core′ of the nucleus whilst its arbor extends radially to synapse with afferent habenular axons that encircle the core. (c, c′) A vIPN neuron with a flattened dendritic arbor. In this specimen two neurons were labeled. One of these (soma marked by arrow in (c′)) radiates an extensive dendritic arbor exclusively on the R of the midline within the vIPN neuropil. The lateral projection (inset in (c′)) reveals the arbor is extremely flattened along the DV axis (arrowheads in (c′)). (d, d′) Neuron with a dorsally located soma (just outside the dIPN neuropil), which elaborates two arbors in spatially distinct regions of the IPN. The larger of these arbors (arrowhead in (d′)) is connected to the cell body (arrow in (d′)) by a long, unbranched process. As is also the case for some of the other interneurons, it is unclear whether these arbors are axonal, dendritic or both in nature. (e, e′) A dIPN projection neuron that extends an axon just caudal to the IPN, most probably synapsing with neurons of the serotonergic raphé nucleus. This neuron has dendritic processes located both in the dorsal (D) and ventral (V) neuropils. (f, f′) In this specimen, three projection neurons are labeled. Axons extend around the IPN (arrowhead in (f)) as well as caudally and dorsally (arrows in (f, f′)). (g) Projection neurons extending anteriorly directed axons that pass around the IPN, cross the midline and continue rostrally (arrow). Scale bar in (a′): 20 μm |
|
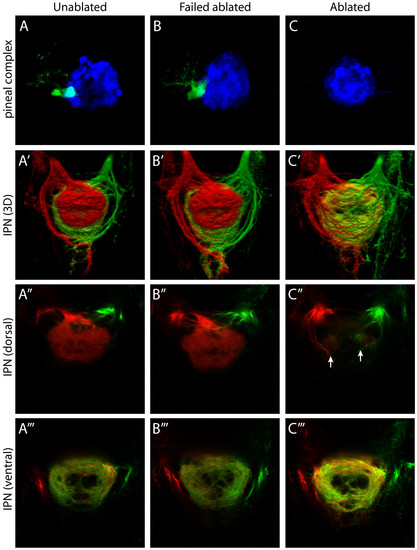
Parapineal ablation eliminates laterotopic habenular efferent connectivity. (a-c) Dorsal views of the pineal complex at 3 dpf, visualized in Tg(flh:eGFP); Tg(foxD3:GFP) transgenic larvae, following laser ablation of parapineal precursor cells at 24–28 hpf. (a) Unablated control larva. (b) Failed ablation control larva, in which the parapineal has not been eliminated. (c) Parapineal-ablated larva, which lacks all parapineal cells. For clarity, pineal cells are pseudocolored blue and parapineal cells pseudocolored green. (a′-c′) Three-dimensional reconstructions of habenular axon terminals in the IPN following lipophilic dye labeling of L (red) and R (green) habenular neurons. (a″, b″, c″) Single-depth confocal images through the dorsal part of the IPN. (a″′, b″′, c″′) Single-depth confocal images through the ventral part of the IPN. Following parapineal ablation there is an almost complete loss of L habenular innervation of the dIPN (c″). However, two small tufts of neuropil, containing both L- and R-sided axons, are consistently observed in the rostral dIPN (arrows in (c″)). In parapineal-ablated larvae there is an increase in the density of L-sided axon terminals in the vIPN (compare (c″′) to (a″′) and (b″′)). All panels show dorsal views, anterior top. EXPRESSION / LABELING:
|
|
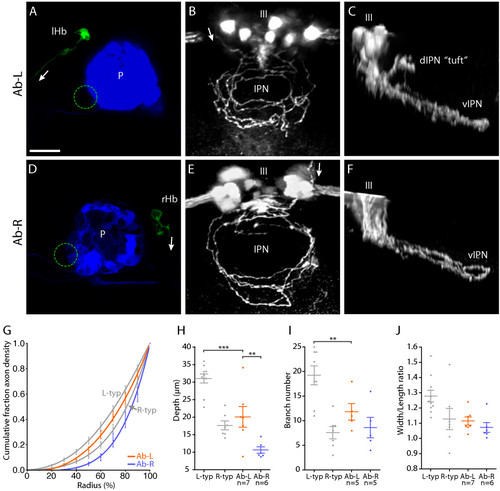
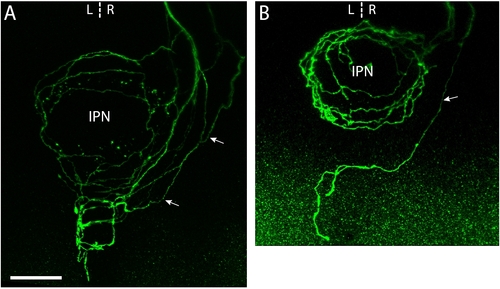
Left and right-sided habenular axons retain distinct morphologies in parapineal-ablated larvae. (a-f) Images from brains of 4 dpf Tg(flh:eGFP); Tg(foxD3:GFP) transgenic larvae in which parapineal ablation was performed at 24–28 hpf and single neurons in the L or R habenula were labeled by focal electroporation at 3 dpf. (a, d) Confocal z-projections of the dorsal diencepahlon confirming successful ablation of the parapineal (indicated by dotted circles) and labeling of single L (a) or R (d) habenular (Hb) projection neurons. 'P' indicates pineal. (b, c) Dorsal (b) and lateral (c) views of flattened axonal arbors elaborated by single L-sided neurons in the vIPN after parapineal ablation. Axon branches frequently extend towards the center of the vIPN in these arbors. Some L-sided axons extend collateral branches into the anterior dIPN that terminate with a unique tuft morphology (tuft in (c)). The oculomotor nucleus (III), which lies just anterior to the IPN, expresses GFP in these transgenic larvae and allows the DV position of the arbors to be determined. (e, f) Dorsal (e) and lateral (f) views of arbors formed by single R-sided neurons after parapineal ablation. These arbors appear as a more exaggerated form of the R-typical morphology. Axon branches are strongly localized to the perimeter of the arbor and extend over a very limited DV depth. Arrows indicate the direction of projection of habenular axons. Scale bar in (a): 20 μm. (g) Radial distribution of axon density for six Ab-L and five Ab-R arbors. Distribution profiles for L-typical (L-typ) and R-typical (R-typ) arbors are shown in grey. (h) Both Ab-L and Ab-R arbors extend over a limited DV depth, similar to R-typical arbors; however, Ab-R arbors are significantly flatter than Ab-L arbors. (i) Ab-L arbors have a reduced number of branch points compared to L-typical arbors. (j) The width/length ratio of Ab-L and Ab-R arbors are similar to those of R-typical arbors. In (g-j) the L-typical and R-typical data, shown in grey, are the same as presented in Figure 3. Horizontal lines indicate mean values and error bars show standard error of the mean. **p < 0.01; ***p < 0.001. |
|
A subset of habenular projection neurons extend axons that pass around the IPN and terminate in the anterior hindbrain. (a, b) Confocal z-projections of the ventral midbrain and anterior hindbrain in 5 dpf (a) and 8 dpf (b) larvae in which groups of habenular neurons have been labeled by focal electroporation. Some habenular neurons project axons that course ipsilaterally around the IPN (arrows) before converging medially to terminate on either side of the midline. These caudal terminations lie in the anterior hindbrain at the level of the serotoninergic raphé nucleus [16]. Scale bar: 25 μm. |
|
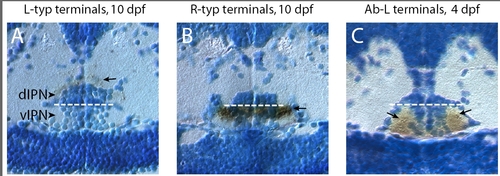
Localization of habenular axon arbors to the dIPN or vIPN. (a-c) Transverse plastic sections through the IPN of larvae in which habenular neurons were labeled by focal electroporation, followed by anti-GFP immunostaining (brown) to determine the location of axon arbors (indicated by arrows). (a) L-typical arbors are localized in the neuropil surrounding and covering the dIPN. (b, c) By contrast, R-typical terminals (b) and Ab-L terminals (c) are located in the vIPN. For (c), the parapineal ablation was performed in a Tg(flh:eGFP); Tg(foxD3:GFP) transgenic embryo in which GFP is weakly expressed in the habenular axons innervating the vIPN. The strongly labeled Ab-L terminals are seen as dark puncta (arrows in (c)) within the more lightly stained vIPN neuropil. Panels show transverse sections through 10 dpf (a, b) or 4 dpf (c) larval brains. Dotted white lines indicate the boundary between the dorsal and ventral parts of the IPN. |
|
Parapineal ablation causes a substantial reduction in epithalamic asymmetries. (a-f) Dorsal views of the epithalamus in larvae in which parapineal ablation was performed at 24–28 hpf, and gene expression and neuropil organization were assessed at 4 dpf. (a, b) In the parapineal-ablated larva, lov expression is substantially reduced in the L habenula to levels similar to the R habenula. However, a small, asymmetric, medial expression domain is retained (arrow in (b)). (c, d) ron expression appears bilaterally symmetric in the parapineal-ablated larva. (e, f) Anti-acetylated tubulin immunostaining reveals a considerable reduction in the size of the asymmetric dorsomedial neuropil domain in the L habenula after parapineal ablation. However, a small medial 'stump' is retained (arrows). All panels show dorsal views, anterior top. EXPRESSION / LABELING:
|