- Title
-
Apigenin promotes melanogenesis and melanosome transport through the c-KIT/Raf-1/MAPK/CREB pathway in HEMCs
- Authors
- Lv, J., Meng, D., Zhang, H., Xu, W., An, X., Yin, C., Zou, K., Gao, R.
- Source
- Full text @ Front Pharmacol
|
Apigenin induces hyperpigmentation in melanocytes. |
|
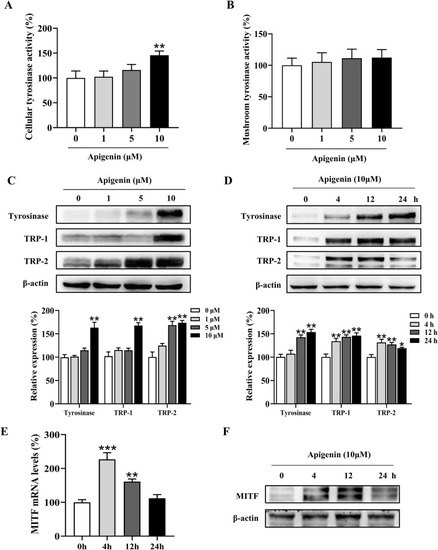
Apigenin increases cellular tyrosinase activity and expression of melanogenic proteins. |
|
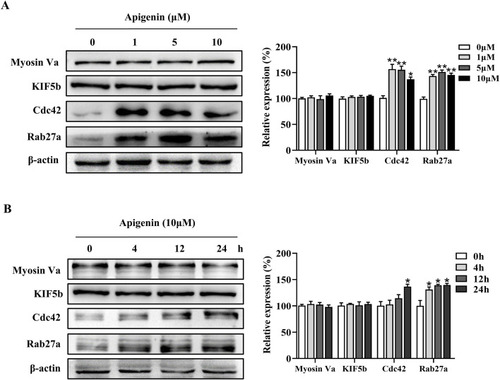
Apigenin promotes the expression of melanosome transport proteins in HEMCs. |
|
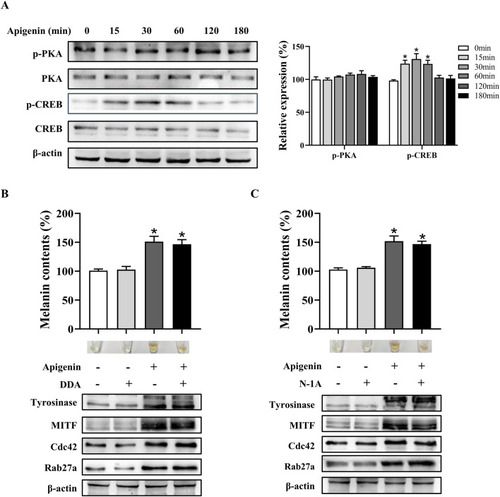
Apigenin promotes pigmentation independent of the classic MC1R/cAMP/PKA-pigmentation pathway in HEMCs. |
|
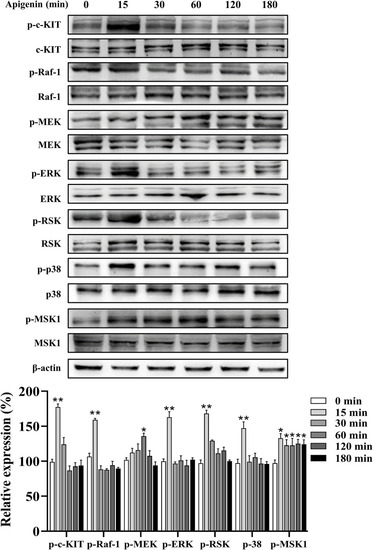
Apigenin activates the c-KIT/Raf-1/MAPK signaling pathway in HEMCs. HEMCs were treated with apigenin (10 μM) for the indicated time periods (0–180 min), and the phosphorylation levels of c-KIT, Raf-1, MEK, ERK, RSK, p38, and MSK1 were assessed by Western blotting. Data are expressed as means ± SEM (n = 3). *p < 0.05, **p < 0.01 vs. untreated cells. c-KIT, cellular-KIT; Raf-1, rapidly accelerated fibrosarcoma-1; MAPK, mitogen-activated protein kinase; HEMCs, human epidermal melanocytes; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; RSK, p90 ribosomal S6 kinase; p38, p38 mitogen-activated protein kinase; MSK1, mitogen-and stress-activated protein kinase 1. |
|
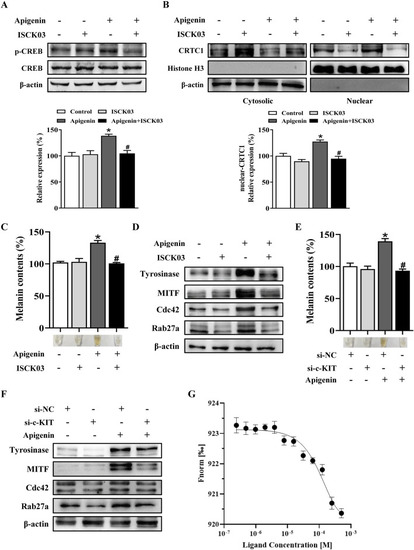
Apigenin promotes pigmentation through the c-KIT-CRTCs/CREB signaling pathway. |
|
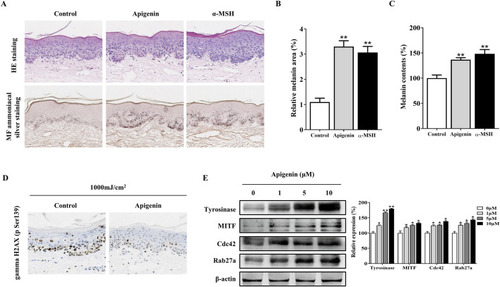
Apigenin increases pigmentation in zebrafish. |
|
Apigenin induces pigmentation in human skin explants. |
|
A proposed model showing that apigenin promotes pigmentation through the c-KIT/Raf-1/MAPK/CREB signaling pathway. Apigenin activates the c-KIT receptor, leading to phosphorylation of Raf-1. Once activated, Raf-1 phosphorylates and activates MAPK, resulting in CREB phosphorylation and subsequent nuclear translocation of CRTCs. Phosphorylated CREB, in association with CRTCs, promotes MITF transcription, which in turn induces the expression of Tyrosinase, Rab27a, and Cdc42. This cascade of events drives melanosome maturation and transport. Tyrosinase, TRP-1, and TRP-2 are key regulators of melanosome maturation. Cdc42 facilitates dendrite extension and filopodia formation, while Rab27a interacts with its effectors, Mlph and Myosin Va, to regulate actin-dependent melanosome transport and anchoring to the plasma membrane. c-KIT, cellular-KIT; Raf-1, rapidly accelerated fibrosarcoma-1; MAPK, mitogen-activated protein kinase; CRTC, CREB-regulated co-activator; CREB, cAMP response element-binding protein; MITF, Melanocytes inducing transcription factor; Cdc42, cell division cycle 42; Rab27a, Ras-related protein Rab-27a; TRP-1, tyrosinase-related protein-1; TRP-2, tyrosinase-related protein-2. |