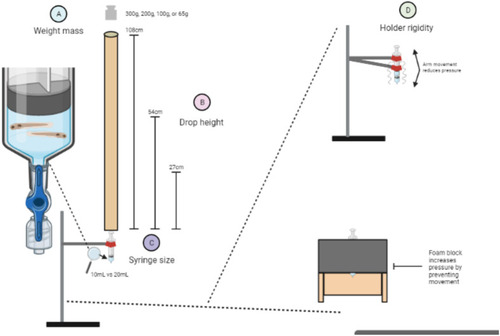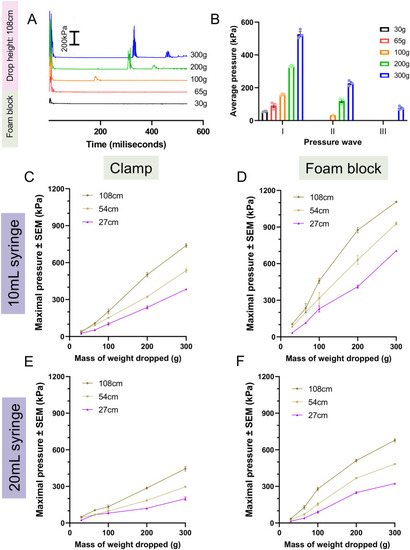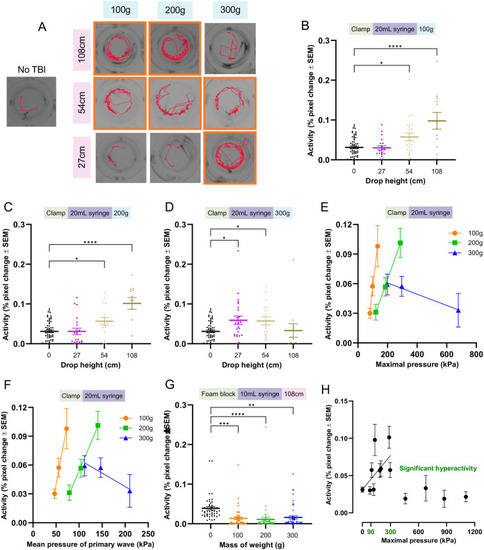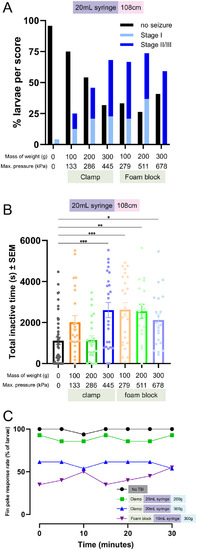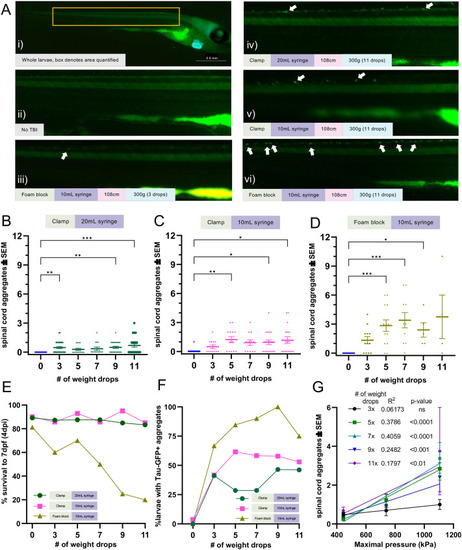
Tau aggregation increases with injury levels. Tau biosensor larvae were used to quantify how increasing injury intensity impacts the amount of detectable tau pathology. These transgenic larvae express full-length human tau and the human tau 4R domain linked to GFP, allowing for the visualization of tau aggregates as GFP+ puncta. Data represents individual larvae and bars represent +/- SEM and each experimental group was replicated at least twice. (A) Representative images of larval zebrafish spines, where quantifiable GFP+ Tau puncta increase as the severity of injury and number of weight drops increase. Image i depicts the full body of a larva for orientation and scale (scale bar: 0.5 mm). White arrows denote GFP+ Tau puncta. (B) Quantification of GFP+ tau aggregates in larvae injured in a 20mL syringe held by a clamp (N=41, 34, 14, 14, 26, and 24 for the 0, 3, 5, 7, 9 and 11 drops respectively). (C) Quantification of GFP+ tau aggregates in larvae injured in a 10mL syringe held by a clamp (N=27, 12, 13, 12, 19, and 18 for the 0, 3, 5, 7, 9 and 11 drops respectively). (D) Quantification of GFP+ tau aggregates in larvae injured in a 10mL syringe held by a foam block (N=13, 12, 13, 10, 5, and 4 for the 0, 3, 5, 7, 9 and 11 drops respectively). (E) Percent of zebrafish larvae that survived to 7 dpf after TBI using each TBI setup (N=equivalent to B-D). (F) Percent of zebrafish larvae with one or more tau GFP+ puncta in each TBI model setup. (G) Linear regression of mean spinal cord tau puncta (data from Fig. 5B-D) as a function of maximal pressure for larval injury groups that received differing numbers of weight drops. Every weight drop group except the three weight drop group significantly trended with maximal pressure. The five and seven weight drop groups had the highest R2.
|