- Title
-
Disrupting USP39 deubiquitinase function impairs the survival and migration of multiple myeloma cells through ZEB1 degradation
- Authors
- Sirera, J., Sarlak, S., Teisseire, M., Carminati, A., Nicolini, V.J., Savy, C., Brest, P., Juel, T., Bontoux, C., Deckert, M., Ohanna, M., Giuliano, S., Dufies, M., Pages, G., Luciano, F.
- Source
- Full text @ J. Exp. Clin. Cancer Res.
|
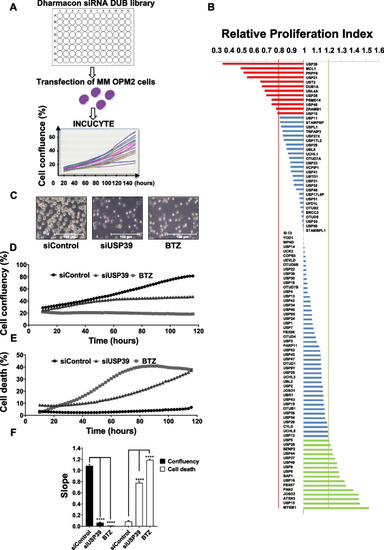
The depletion of USP39 suppresses proliferation and induces cell death in OPM2 myeloma cell line |
|
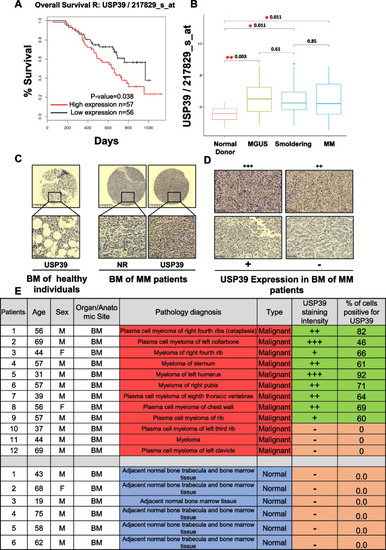
USP39 is overexpressed in MM patients compared to healthy donors and its high expression is correlated with shorter survival. |
|
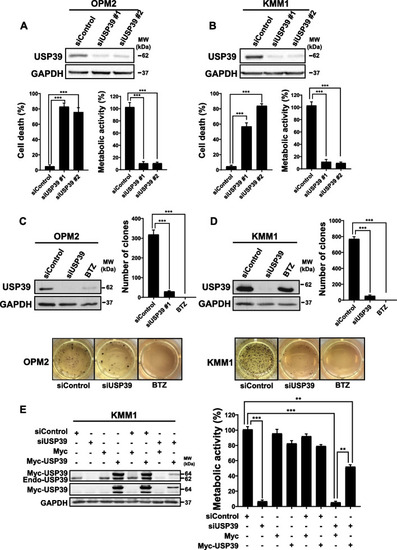
USP39 Depletion Suppresses Cell Proliferation, Induces Apoptosis, and decreases Clonogenicity in OPM2 and KMM1 Multiple Myeloma Cells. |
|
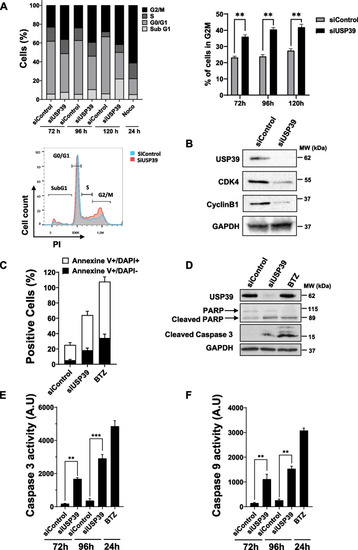
Inhibition of USP39 Triggers G2/M Cell Cycle Arrest and Apoptosis in Multiple Myeloma Cells. |
|
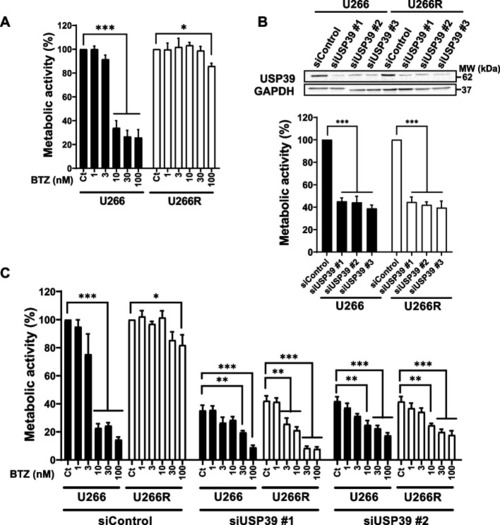
USP39 Inhibition Overcomes Bortezomib Resistance in MM Cells. |
|
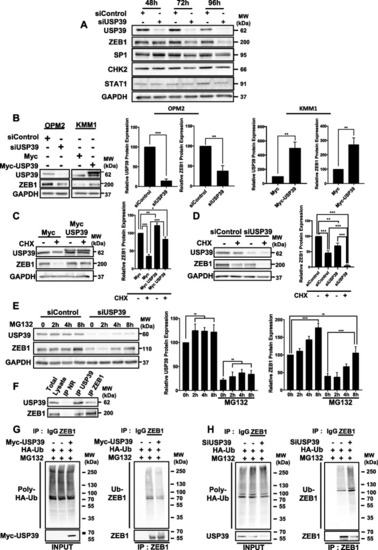
USP39 Stabilizes and Deubiquitinates ZEB1 Protein in Multiple Myeloma Cells |
|
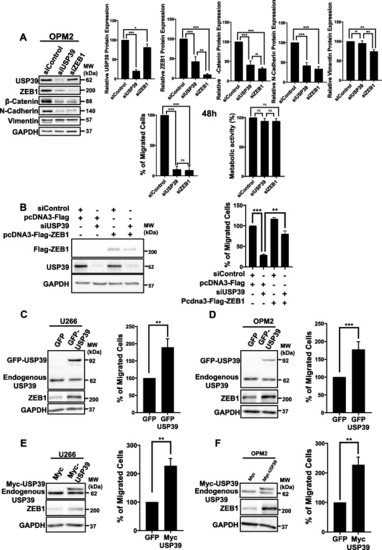
USP39 Promotes In Vitro Transmigration of MM Cells. |
|
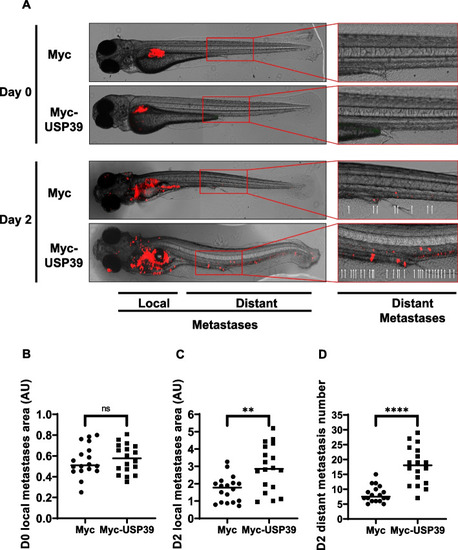
USP39 Enhances Metastasis in Zebrafish: Implications for MM Progression. |