- Title
-
TFAP2E is implicated in central nervous system, orofacial and maxillofacial anomalies
- Authors
- Kalanithy, J.C., Mingardo, E., Stegmann, J.D., Dhakar, R., Dakal, T.C., Rosenfeld, J.A., Tan, W.H., Coury, S.A., Woerner, A.C., Sebastian, J., Levy, P.A., Fleming, L.R., Waffenschmidt, L., Lindenberg, T.T., Yilmaz, Ö., Channab, K., Babra, B.K., Christ, A., Eiberger, B., Hölzel, S., Vidic, C., Häberlein, F., Ishorst, N., Rodriguez-Gatica, J.E., Pezeshkpoor, B., Kupczyk, P.A., Vanakker, O.M., Loddo, S., Novelli, A., Dentici, M.L., Becker, A., Thiele, H., Posey, J.E., Lupski, J.R., Hilger, A.C., Reutter, H.M., Merz, W.M., Dworschak, G.C., Odermatt, B.
- Source
- Full text @ J. Med. Genet.
|
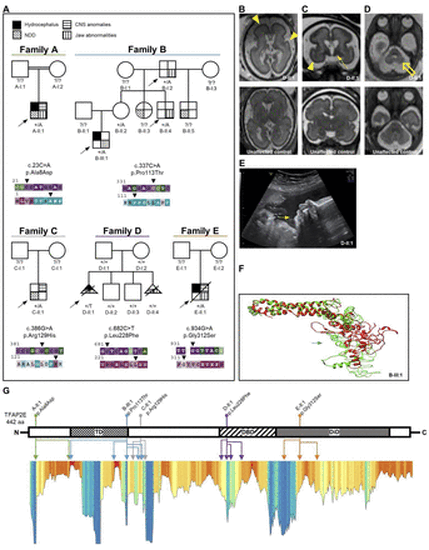
Clinical and molecular data of families with heterozygous transcription factor activator protein 2 (TFAP2E) missense variants. (A) Family pedigrees of individuals with heterozygous variants in TFAP2E and respective ConSurf conservation analysis for base pair (upper row) and amino acid (aa) conservation (lower row). Affected individuals are indicated with different shapes for each phenotype according to the legend. Arrowheads indicate the respective variant position. Purple/red colour indicates high conservation of the respective residue. Note the multiplex family B with overlapping orofacial and maxillofacial and neurodevelopmental phenotype (table 1, online supplemental data 1) and the de novo variant in the female individual D-II:1. The arrows indicate probands. Filled shapes reflect affected status. (B–D) Fetal brain MRI of D-II:1 (upper panel) compared with that of a healthy subject with similar gestational age as reference (lower panel). Besides the obvious microcephaly, note the reduced sulcation with smooth brain surface characteristic of lissencephaly (B, C, yellow arrowheads). Furthermore, an overall abnormal signal intensity of subcortical white matter as well as hypotrophy of basal ganglia is evident (C, yellow solid arrow). The suspected cerebellar hypoplasia was seen in the axial plane (D, yellow hollow arrow). (B) Axial T2 TSE (turbo spin echo), (C) coronal SSFP (steady-state free precession), (D) axial SSFP. (E) Ultrasound imaging of fetus D-II:1 at gestational week 30+4 shows severe retrognathia (white arrow). (F) Computational 3D protein modelling of TFAP2E showing the native prediction (green) and the variant prediction of B-III:1 (red). The variant site is marked by the respective (green/red) arrow. The missense variant changes the polarity of the aa residue, leading to conformation changes of the neighbouring aa. This completely changes the 3D structure of the affected transactivation domain. (G) Linearised visualisation of the TFAP2E protein and lollipop chart indicating the localisation of the individuals’ aa variants, which are colour-coded according to the family pedigrees in A. The identically colour-coded double-headed arrows below indicate the multiple positions of the variants’ structural effects predicted by our protein modelling data. Lower panel: Metadome Protein Tolerance Landscape indicating the residues with general intolerance to structural variations (orange–red colour indicates high intolerance). Note that for some variants a secondary effect to a neighbouring position and not the variant location itself are in a region of strong intolerance. That is true for p.Ala8Asp (A-II:1), p.Pro113Thr (B-III:1) and p.Leu228Phe (D-II:1) (online supplemental table 1). CNS, central nervous system; DBD, DNA-binding domain; DiD, dimerisation domain; NDD neurodevelopmental disorder; TD transactivation domain. |
|
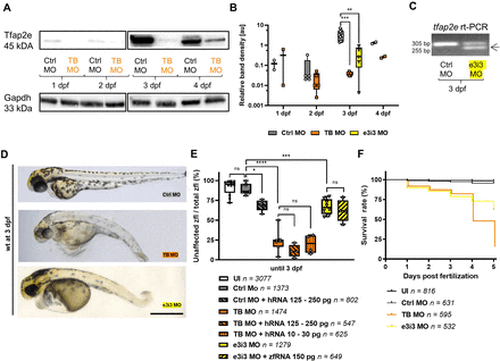
Tfap2e morpholino KD model in zfl. (A–B) Chemiluminescence bands and box plot graph of western blots of zfl on Tfap2e morpholino (MO) KD and controls from 1–4 days post fertilisation (dpf). Each dot on the box plot graph represents an independent experiment with 50 µg zfl protein of an independent injection. Band intensities were plotted on a logarithmic scale. Note the increasing genuine Tfap2e expression between 2 dpf and 3 dpf and the significantly suppressed expression on TB and e3i3 MO KD at 3 dpf. The housekeeping control Gapdh shows a consistent expression over all timepoints. The uncropped western blot membrane pictures are provided in online supplemental data 11. (C) Agarose gel electrophoresis after rt-PCR on tfap2e at 3 dpf. Tfap2e KD with e3i3 MO leads to an alternative, 50 bp shorter transcript (arrow) with precise exclusion of exon 3. This was further confirmed by Sanger sequencing (online supplemental data 7). (D–E) Representative brightfield microscopy images of zfl and box plot graph on the rate of unaffected zfl on Tfap2e KD. Each dot in the box plot graph indicates an independent injection experiment. Wild type (wt) strain zfl on tfap2e MO KD at 3 dpf present with microcephaly, scoliosis and hypopigmentation. The KD results in a significant reduction of phenotypically unaffected zfl. Co-injection of wt human RNA or zebrafish RNA (zfRNA) in morphants does not rescue the phenotype. Moreover, overexpression of TFAP2E by wt TFAP2E human polyA mRNA (hRNA) injection into Ctrl MO zfl leads to expression of the morphant phenotype in some of these controls. (F) Kaplan-Meier survival curve of tfap2e MO KD zfl. Both morphants show a poorer survival rate with a rapidly decreasing survival after 3 dpf especially for the TB MO KD, congruent with the timepoint of physiological Tfap2e upregulation. n=4; p<0.0001 (Mantel-Cox test). Ns, not significant; *p<0.05 **; p<0.01; ***p<0.001; ****p<0.0001 (B, E: one-way analysis of variance (ANOVA) with Tukey’s multiple comparison). Number of investigated zfl n as indicated. Scale bar: 500 µm. KD, knockdown; TB, translation blocking; TFAP2E, transcription factor activator protein 2; zfl, zebrafish larvae. |
|
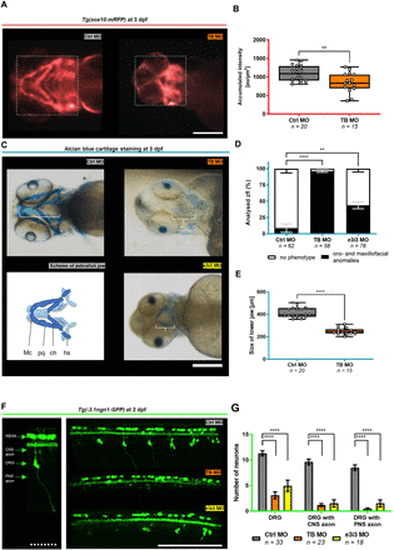
Tfap2e KD is implicated in the disruption of neural crest-specific tissues. (A–B) Representative in vivo fluorescence imaging of the jaw of Tg(sox10:mRFP) zfl at 3 days post fertilisation (dpf) on Tfap2e KD and box plot graph of sox10:mRFP reporter-signal intensity. The dotted rectangle indicates the area that was chosen to measure the accumulated fluorescence intensity in arbitrary units (au)/µm2. Morphants present with a pathological formation and a reduced Sox10 signal at 3 dpf after Tfap2e KD, indicating neural crest cell disruption. (C–E) Representative Alcian blue cartilage staining of zfl at 3 dpf and simplified schematic of the developing zebrafish craniofacies (C), column graph of the percentage of zfl with orofacial and maxillofacial anomalies (D), and box plot graph of measured sizes of the lower jaw on Tfap2e KD (E). White brackets in (C) indicate the measured distance from the rostral end of the Meckel’s cartilage to the caudal point of the hyosympletic cartilage. On Tfap2e KD, we observed a reduced jaw size according to hypoplasia of relevant orofacial and maxillofacial structures including the Meckel’s cartilage (Mc), ceratohyal (ch), palatoquadrate (pq) and hyosympletic (hs). (F–G) Representative in vivo 2-photon microscopy of the spinal cord of Tg(−3.1ngn1:GFP) zfl on Tfap2e KD at 2 dpf and column graph of counted fluorescent neurons and their axons. The left panel is a close-up view and description of the analysed cell types. Note the strongly reduced number of DRG neurons in both morpholino (MO) KD groups. Additionally, central and peripheral axonal outgrowth of the remaining DRG neurons is impaired. **p<0.01, ****p<0.0001 (B, E: unpaired t-test; D: one-way ANOVA with Tukey’s multiple comparison; G: two-way ANOVA with Tukey’s multiple comparison). Number of independent experiments n=3 for all graphs, number of investigated zfl n as indicated. White scale bars: 200 µm. White dotted scale bar: 50 µm. ANOVA, analysis of variance; CNS, central nervous system; DRG, dorsal root ganglia; KD, knockdown; TB, translation blocking; zfl, zebrafish larvae. PHENOTYPE:
|
|
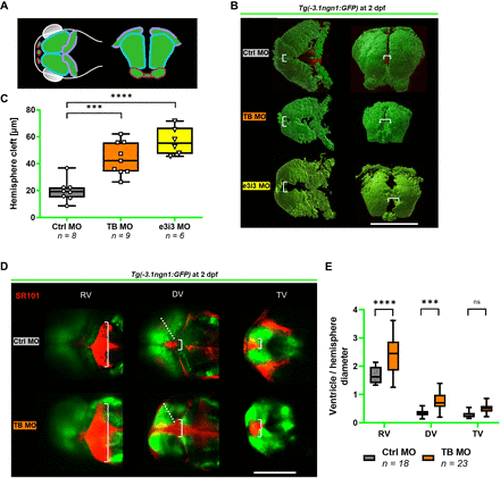
Tfap2e KD is implicated in structural brain anomalies in the early developing zfl. (A–C) 3D modelling of the brain of Tg(−3.1ngn1:GFP) zfl on Tfap2e KD at 2 days post fertilisation (dpf) and box plot graph of the forebrain hemisphere cleft measured as indicated (white brackets). (A) shows a schematic depiction of zfl brain in transverse (left) and coronary view (right) (modified from Dworschak et al, 2021).28 Pink: olfactory blub. Turquoise: mesencephalon. Purple: rhombencephalon. (B) The representative images were obtained by in vivo 2-photon z–stack imaging and subsequent modulation with IMARIS software. Transverse (left) and coronary view (right). (C) TB MO and e3i3 morphant zfl present with an enlarged hemisphere cleft consistent with a hydrocephalus. Note that only TB MO but not e3i3 MO treated zfl show a reduced brain volume (see online supplemental data 13). (D–E) Representative confocal in vivo images of Tg(−3.1ngn1:GFP) zfl at 2 dpf in transverse view after sulforhodamine SR101 injection (in red) into the diencephalic ventricle and box plot graph of ventricle diameters (white brackets) in relation to hemisphere diameter (dotted line). In TB MO zfl, both the diencephalic and the rhombencephalic ventricle are enlarged in the sense of a hydrocephalus. ***p<0.001, ****p<0.0001 (C: one-way ANOVA with Tukey’s multiple comparison; E: two-way ANOVA with Tukey’s multiple comparison). Number of independent experiments n=3 for all graphs, number of investigated zfl n as indicated. White scale bars: 200 µm. ANOVA, analysis of variance; DV, diencephalic ventricle; KD, knockdown; MO, morpholino oligonucleotide; ns, not significant; RV, rhombencephalic ventricle; TB, translation blocking; TV telencephalic ventricle; zfl, zebrafish larvae. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|