- Title
-
Zebrafish arterial valve development occurs through direct differentiation of second heart field progenitors
- Authors
- Derrick, C.J., Eley, L., Alqahtani, A., Henderson, D.J., Chaudhry, B.
- Source
- Full text @ Cardiovasc. Res.
|
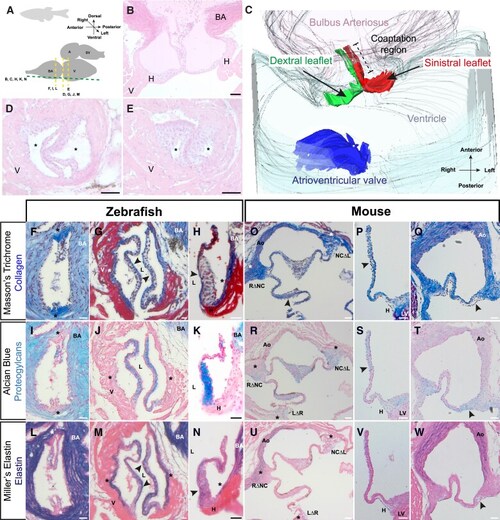
The zebrafish arterial valve is anatomically similar to other vertebrate arterial valves. ( |
|
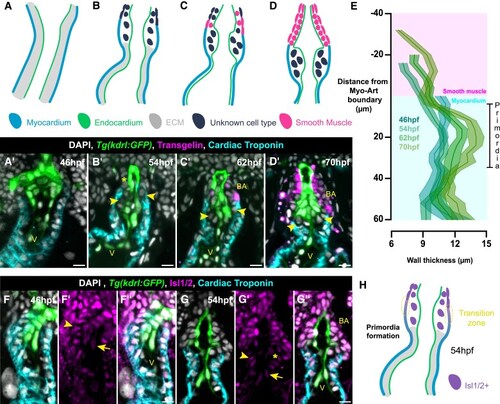
Development of the zebrafish arterial valve follows conserved events. ( |
|
Arterial valve primordia form at the transition zone. ( |
|
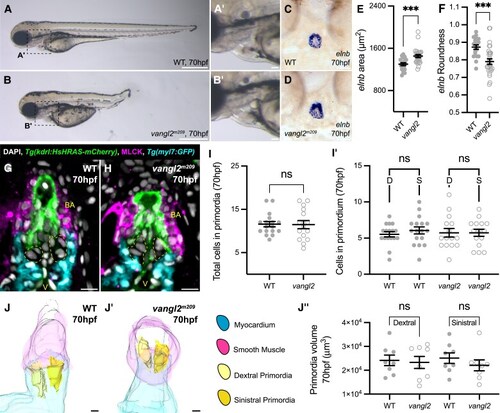
Direct differentiation of SHF progenitors establishes the zebrafish arterial valve. ( |
|
Arterial valve primordia cells are distinct from smooth muscle cells. ( |
|
|
|
|
|
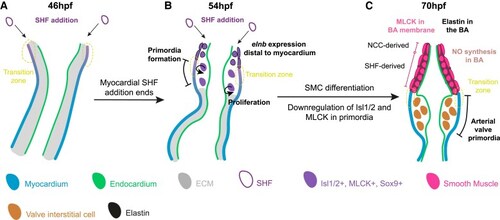
The zebrafish arterial valve forms by direct differentiation of SHF progenitors. Model of zebrafish arterial valve formation. ( |