- Title
-
Drivers of Vessel Progenitor Fate Define Intermediate Mesoderm Dimensions by Inhibiting Kidney Progenitor Specification
- Authors
- Perens, E.A., Yelon, D.
- Source
- Full text @ Dev. Biol.
|
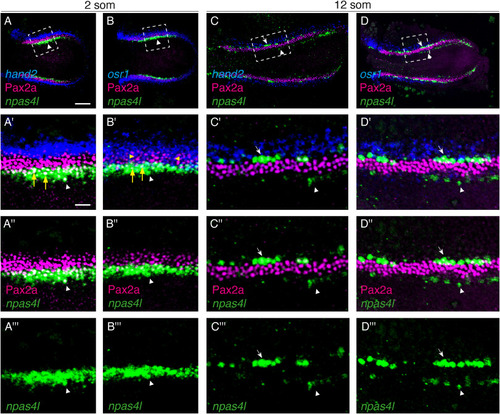
npas4l is expressed at the medial and lateral boundaries of the IM. In situ hybridization chain reaction (A,C: npas4l, hand2; B,D: npas4l, osr1) and immunohistochemistry (Pax2a) label components of the wild-type posterior lateral mesoderm in dorsal views, anterior to the left, of three-dimensional reconstructions at 2 som (A,B) and 12 som (C,D). (A′-D‴) Magnification of boxed regions in A-D. (A-A‴) At 2 som, npas4l is expressed in bilateral territories (green, arrowhead) medial to the IM marker Pax2a (magenta) and hand2 (blue). There is overlap between npas4l expression and Pax2a localization (yellow arrows) seen in A'. (B-B‴) At 2 som, npas4l is expressed in bilateral territories (arrowhead) medial to Pax2a and osr1. There is overlap between osr1 expression and Pax2a localization (yellow arrowheads), as well as overlap of between npas4l expression and Pax2a localization (yellow arrows) in B'. (C-C‴) At 12 som, npas4l-expressing lateral vessel progenitors (arrow) have arisen lateral to Pax2a and medial to hand2. Faint npas4l expression remains in the medial territory (arrowhead). (D-D‴) At 12 som, npas4l-expressing lateral vessel progenitors (arrow) are lateral to Pax2a and medial to osr1. Faint npas4l expression remains in the medial territory (arrowhead). Unlike at 2 som (B′), no substantial osr1 expression is detected in the Pax2a territory, but osr1 expression is still present lateral to Pax2a. Scale bars: A–D: 200 μm; A′-D‴: 50 μm. |
|
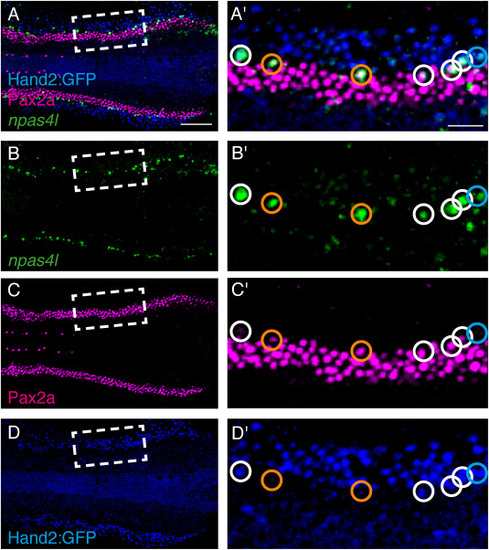
npas4l, Pax2a, and hand2 can be co-expressed in lateral vessel progenitors. In situ hybridization chain reaction for npas4l and immunohistochemistry for Pax2a and GFP in wild-type embryos carrying Tg (hand2:EGFP); dorsal views, anterior to the left, of three-dimensional reconstructions at 12 som. (A′-D′) Magnification of boxed regions in A-D. Circles indicate npas4l-expressing LVPs. White circles denote LVPs with Pax2a and GFP localization; orange circles denote LVPs with Pax2a localization; and cyan circle denotes LVP with GFP localization. Scale bars: A–D: 100 μm; A′-D': 30 μm. |
|
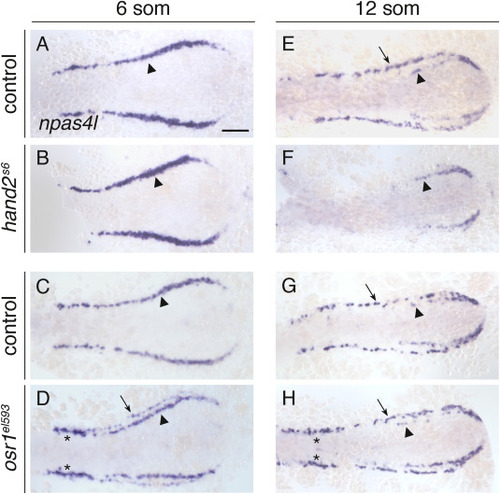
hand2 and osr1 regulate the development of npas4l-expressing lateral vessel progenitors. In situ hybridization shows npas4l expression in wild-type control (A,C,E,G), hand2s6 mutant (B,F) and osr1el593 mutant (D,H) embryos; dorsal views, anterior to the left, at 6 som (A–D) and 12 som (E–F). (A–D) At 6 som, npas4l is expressed in a relatively medial territory (arrowhead) on each side of the wild-type (A,C), hand2s6 (B), and osr1el593 (D) posterior mesoderm. In osr1el593 mutants, npas4l is also expressed in relatively lateral cells (D, arrow), and its expression is increased in a distinct proximal region (D, asterisks). Lateral npas4l expression was observed in 84% of osr1el593 mutants (n = 31) and 2% of wild-type control siblings (n = 110); furthermore, the lateral expression observed in wild-type embryos was restricted to only a few cells at the anterior extent of the posterior mesoderm (Fig. S2C). Similarly premature expression of etv2 in lateral cells was seen in osr1 mutants previously (Perens et al., 2021). (E–H) At 12 som, npas4l is expressed in a relatively lateral territory (arrows) on each side of wild-type (E,G) and osr1el593 mutant (H) embryos; in osr1el593 embryos, npas4l expression remains increased proximally (H, asterisks). In hand2s6 mutants (F), npas4l is not expressed in this lateral territory (100%, n = 20). At this stage, a small amount of npas4l expression is still observed in the posterior medial vessel progenitors in hand2s6 mutants (H, arrowhead). n = 77 (A), 30 (B), 110 (C), 31 (D), 66 (E), 20 (F), 53 (G), 18 (H). Scale bar: 100 μm. |
|
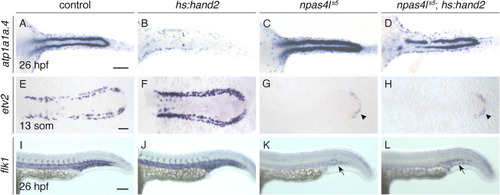
hand2 overexpression requires npas4l function to inhibit pronephron formation. In situ hybridization demonstrates atp1a1a.4 (A–D) expression throughout the pronephron tubules, etv2 (E–H) expression in vessel progenitors, and flk1 (I–L) expression in the vasculature. Dorsal views, anterior to the left, of wild-type (B,F,J) and npas4ls5 mutant embryos (D,H,L) carrying hs:hand2 were compared with their corresponding wild-type control (A,E,I) and npas4ls5 mutant (C,G,K) nontransgenic siblings. Heat shock was performed at tailbud in all embryos shown. Compared to wild-type control embryos (A), atp1a1a.4 expression in the pronephron tubules was expanded in npas4ls5 mutants (C; n = 17/19). Unlike the dramatically decreased atp1a1a.4 expression in hs:hand2 embryos (B), atp1a1a.4 expression in the pronephron of npas4ls5;hs:hand2 embryos is only relatively mildly reduced (D; n = 34/38). Compared to wild-type (E), etv2 expression appears more intense and is observed in an increased number of cells, especially within the territory normally occupied by the IM, in hs:hand2 embryos (F). etv2 (G,H) and flk1 (K,L) expression are largely absent in both npas4ls5 mutants (G,K), and npas4ls5;hs:hand2 embryos (H,L). Note the faint etv2 expression in the tailbud of the npas4ls5 mutants (arrowheads) and flk1 expression in the posterior trunk of the npas4ls5 mutants (arrows) are consistent with prior reported phenotypes of npas4l mutants (Liao et al., 1997; Reischauer et al., 2016; Sumanas et al., 2005). n = 65 (A), 129 (B), 19 (C), 38 (D), 119 (E), 118 (F), 37 (G), 33 (H), 19 (I), 8 (K), 6 (L). Scale bars: 100 μm. |
|
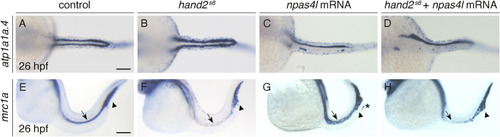
npas4l overexpression inhibits pronephron formation in the absence of hand2 function. Dorsal views, anterior to the left, of wild-type control embryos (A,E), hand2s6 mutant embryos (B,F), and wild-type control (C,G) and hand2s6 mutant embryos (D,H) injected with npas4l mRNA, all at 26 hpf. In situ hybridization demonstrates atp1a1a.4 (A–D) expression throughout the pronephron tubules, and mrc1a (E–H) expression in the posterior cardinal vein (arrow) and posterior blood island (arrowhead) (Stachura and Traver, 2016). Compared to wild-type (A), pronephron gene expression is expanded in hand2s6 mutants (B) and greatly reduced in npas4l-overexpressing embryos (C,D). The extent of the defects seen in npas4l-overexpressing embryos was comparable between injected hand2s6 mutants (D; n = 10/28 with reduced pronephron as shown) and injected wild-type control embryos (C; n = 26/79 with reduced pronephron as shown). Compared to wild-type (E) and npas4l-overexpressing (G) embryos, mrc1a gene expression in the posterior cardinal vein (arrow) is greatly reduced in both uninjected (F) and npas4l-overexpressing (H) hand2s6 mutants. npas4l mRNA overexpression also resulted in ectopic mrc1a expression along the yolk extension in some injected animals (asterisk in G; ectopic expression in injected wild-type 41/75, in injected hand2s6 10/19). Although higher doses of npas4l mRNA led to stronger phenotypes than shown here, they also increased embryonic lethality or severe deformation. Therefore, the dose of npas4l mRNA injected was limited to 5–14.5 ng so that overall development was not severely impacted which likely limited the penetrance and expressivity of phenotypes displayed. n = 28 (A), 11 (B), 79 (C), 28 (D), 38 (E), 13 (F), 75 (G), 19 (H). Scale bars: 100 μm. |
|
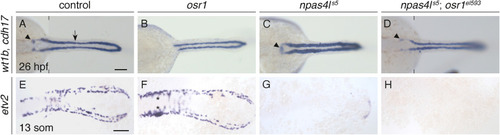
Pronephron defects in osr1el593 mutants are partially suppressed by loss of npas4l function. Dorsal views, anterior to the left, of wild-type control (A,E), osr1el593 mutant (B,F), npas4ls5 mutant (C,G) and npas4ls5;osr1el593 double mutant (D,H) embryos at 26 hpf (A–D) and 13 som (E–H). In situ hybridization shows expression of wt1b in glomerular precursors (arrowhead), cdh17 throughout the pronephron tubules (arrow) (A–D), and etv2 in vessel progenitors (E–H). Compared with wild-type controls (A), pronephron glomerular expression is absent and tubule expression is thin and shortened anteriorly in osr1el593 mutants (B), expanded in npas4ls5 mutants (C); and relatively similar to wild-type in npas4ls5;osr1el593 double mutants (D; n = 8; 75% with normal tubules and wt1b glomeruli expression; 25% with normal tubules and absent wt1b glomeruli expression). In contrast, compared with wild-type controls (E), etv2 vessel progenitor expression is unaffected in osr1 mutants (F; expression was expanded in the midbody region (asterisks), as previously reported (Perens et al., 2021)), but is mostly absent in both npas4ls5 mutants (G) and npas4ls5;osr1el593 double mutants (H). n = 112 (A), 49 (B), 43 (C), 8 (D), 64 (E), 19 (F), 21 (G), 6 (H). Scale bars: 100 μm. |
|
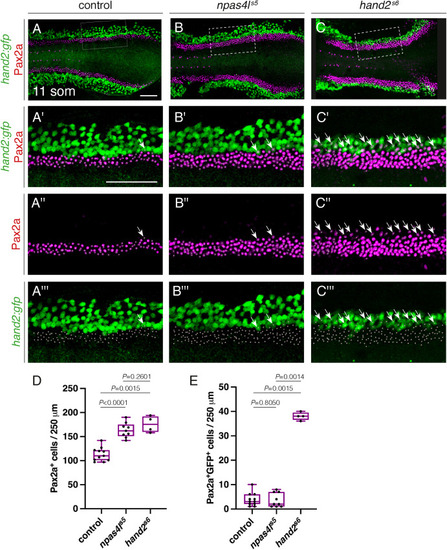
npas4l inhibits IM formation. Immunofluorescence for Pax2a and GFP in wild-type (A), npas4ls5 mutant (B), and hand2s6 mutant (C) embryos carrying Tg (hand2:EGFP); dorsal views, anterior to the left, of three-dimensional reconstructions at 11 som. (A′-C‴) Magnification of regions from (A–C) used for quantification of the numbers of Pax2a+ cells. 250 μm stretches of the IM were analyzed per embryo. The length of the analyzed region varies within the boxed region due to mild curvature of the embryo in the z-axis. White dots indicate Pax2a+ nuclei and arrows indicate examples of Pax2a+GFP+ cells. Scale bars: 100 μm. (D,E) Quantification of the numbers of Pax2a+ cells (D) and Pax2a+GFP+ cells (E) per 250 μm of IM in wild-type control, npas4ls5, and hand2s6 embryos demonstrates an increase in IM cells in npas4ls5and hand2s6 embryos, but an increase in Pax2a+GFP+ cells only in hand2s6 embryos. Boxes represent interquartile range, central line marks the median, and whiskers indicate maximum and minimum values. P values were calculated using non-parametric Mann–Whitney U-tests. n = 11 (A), 9 (B), 4 (C). |
|
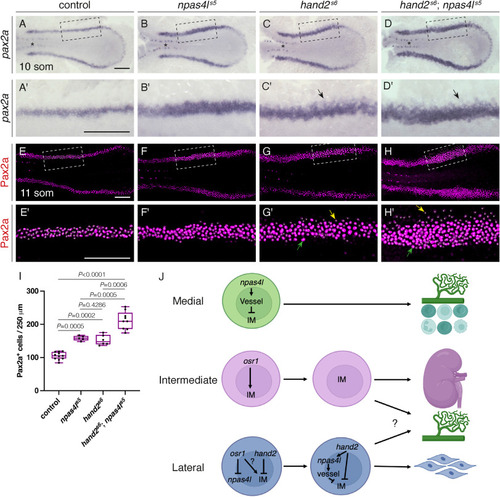
IM defects in npas4ls5mutants are enhanced by loss of hand2 function. (A–H) Dorsal views, anterior to the left, of the posterior mesoderm. (A–D) In situ hybridization shows expression of pax2a in the IM (arrows) at 10 som. Compared with wild-type (A), expression is widened in npas4ls5 mutants (B) and hand2s6 mutants (C) and widened further in hand2s6;npas4ls5 double mutants (D). Expression in the overlying spinal neurons (asterisk) is unaffected. Unlike the width of the IM, we did not observe a noticeable change in the proximal-distal length of the IM in mutant embryos. (A′-D′) Magnification of boxed regions in (A–D). Faint lateral staining (arrows) was frequently observed in hand2s6 mutant (C′) and hand2s6;npas4ls5 double mutant (D′) embryos. n = 60 (A), 17 (B), 21 (C), 7 (D). (E–H) Three-dimensional reconstructions of Pax2a immunofluorescence in the IM of wild-type (E), npas4ls5 mutant (F), hand2s6 mutant (G) and hand2s6;npas4ls5 double mutant (H) embryos at 11 som. (E′-H′) Magnification of boxed 250 μm long regions used for counting Pax2a+ cells. White dots indicate Pax2a+ nuclei. In hand2s6 mutant and hand2s6;npas4ls5 double mutant embryos, intensity of Pax2a+ staining varied from strong (for example, green arrows) to weak (for example, yellow arrows). As seen with in situ hybridization, the fainter Pax2a+ levels were typically observed at the lateral edge in hand2s6 mutants (G′) and hand2s6;npas4ls5 double mutants (H′). n = 11 (E), 5 (F), 6 (G), 9 (H). Scale bars: 100 μm. (I) Quantification of the numbers of Pax2a + cells per 250 μm of IM in the indicated genotypes demonstrates an increase in IM cells in hand2s6; npas4ls5 double mutant embryos exceeding that seen in either single mutant. Boxes represent interquartile range, central line marks the median, and whiskers indicate maximum and minimum values. P values were calculated using non-parametric Mann–Whitney U-tests. (J) Model of organ field formation in the lateral posterior mesoderm in zebrafish. In the most medial territory, npas4l promotes expression of vessel progenitor genes (labeled “vessel”; including etv2, lmo2, and tal1), which inhibit IM. Expression of the kidney progenitor-associated gene pax2a can initially be detected in this region (Fig. 1, S1; not depicted in model), highlighting the importance of inhibiting further IM-associated gene expression. The medial aspect of this territory also gives rise to hematopoietic progenitors. The intermediate territory expresses IM-associated genes (including lhx1a and pax2a); osr1 is initially expressed in this territory (Fig. 1, S1) and is required for forming the full complement of IM (Perens et al., 2021). Initially, the most lateral territory expresses both osr1 and hand2 (Perens et al., 2016). Whether osr1 also promotes formation of some kidney progenitors in this territory remains unknown. Later, as osr1 expression decreases (as shown in (Perens et al., 2021)), hand2 expression promotes npas41 expression, which inhibits further IM gene expression and results in the generation of the LVPs. The precise origin of the LVPs remains unclear as they can express markers of both the intermediate (pax2a) and lateral (hand2) territories (Fig. 2). The remainder of the lateral territory continues to express hand2 and gives rise to other lateral plate mesoderm derivatives, including the mesothelium (Prummel et al., 2022). |
Reprinted from Developmental Biology, 517, Perens, E.A., Yelon, D., Drivers of Vessel Progenitor Fate Define Intermediate Mesoderm Dimensions by Inhibiting Kidney Progenitor Specification, 126-139, Copyright (2024) with permission from Elsevier. Full text @ Dev. Biol.