- Title
-
Alcohol exposure suppresses ribosome biogenesis and causes nucleolar stress in cranial neural crest cells
- Authors
- Flentke, G.R., Wilkie, T.E., Baulch, J., Huang, Y., Smith, S.M.
- Source
- Full text @ PLoS One
|
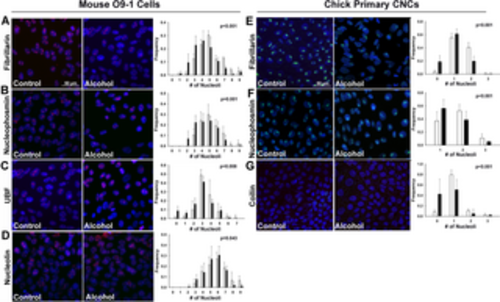
Acute alcohol exposure causes nucleolar stress in the mouse CNC line O9-1 and in primary chick CNCs. (A-D) Murine O9-1 cells were exposed to 60mM ethanol for 2hr and assessed 6hr after the alcohol addition. Compared to untreated cells (Control, left panels), alcohol (middle panel) altered the distribution of nucleoli per nucleus such that there were fewer nucleoli in alcohol-exposed CNCs, as assessed by immunostain for the nucleolar proteins (A) fibrillarin, (B) nucleophosmin, (C) UBF, and (D) nucleolin. Values that quantify the distribution of nucleolar number per nucleus (right panels) are the mean ± SD of three independent experiments as detailed in Methods. (E-G) Primary chick CNCs, derived from cranial explants, were exposed to 60mM ethanol for 2hr and assessed 6hr after the alcohol addition. Compared to untreated cells (left panels), alcohol (middle panels) altered the distribution of nucleoli per nucleus such that there were fewer nucleoli in alcohol-exposed CNCs, as visualized using immunostain for (E) fibrillarin, (F) nucleophosmin, and (G) coilin. Values that quantify the distribution of nucleolar number per nucleus (right panels) are the mean ± SD for N = 3–7 experimental replicates as detailed in Methods. For all, nuclei were imaged using DAPI counterstain and representative images are shown; scale bar represents 50 μm. Data were analyzed using chi-square analysis. |
|
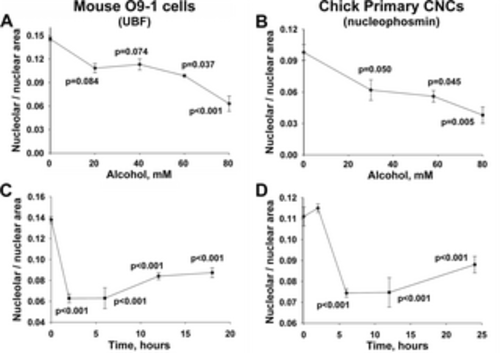
Alcohol-induced nucleolar stress in mouse and chick CNCs is dose-dependent and persistent. (A, B) Dose-dependence of nucleolar stress. Alcohol reduced the nucleolar area per nucleus in a dose-dependent manner, at concentrations as low as 60mM for O9-1 cells (for UBF; A) and 29mM for primary CNCs (for nucleophosmin; B). Values are the mean ± SEM from 4 (O9-1) and 8 (primary CNCs) independent experiments. (C, D) Timecourse of nucleolar stress. The nucleolar reduction is rapid and occurs within 2hr (O9-1; 80mM) and 6hr (primary CNCs; 60mM) following the addition of alcohol at each cell lineage’s calculated EC50. Moreover, the nucleolar abundance remains low in both populations for at least 24hr following the 2hr alcohol exposure. Values are mean ± SEM from 12 (O9-1) and 8 (primary CNCs) independent experiments. For all analyses, nucleoli were visualized using antibodies directed against UBF (O9-1) or nucleophosmin (primary CNCs), and nuclei were visualized using DAPI. Data were analyzed using one-way analysis of variance with Holm-Sidak multiple comparisons. |
|
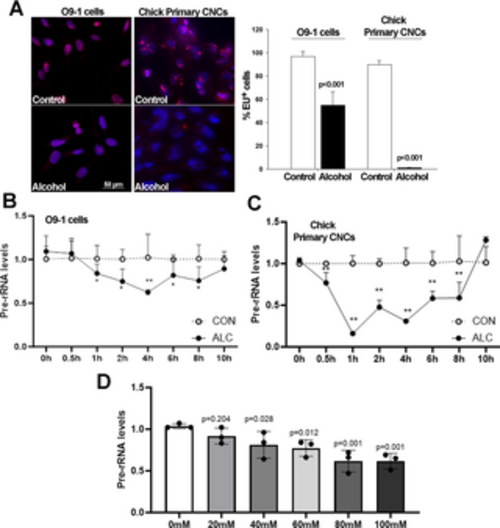
Alcohol suppresses de novo rRNA synthesis and content in O9-1 cells and primary chick CNCs. (A) Alcohol exposure at the EC50 for O9-1 cells (80mM) and chick primary CNCs (52mM) reveals fewer or no EU-positive nuclei (red) in alcohol-exposed nuclei (blue) as compared with controls. Nuclei visualized using DAPI; bar indicates 50 μm. (B) Dose-response shows a significant decline in de novo rRNA synthesis, quantified using qPCR for ITS-1, at alcohol exposures at or above 40mM for O9-1 cells. (C) At 30min following exposure to 80mM alcohol there is a significant decline in ITS-1 content in O9-1 cells. (D) At 30min following exposure to 52mM alcohol there is a significant decline in ITS-1 content in chick primary CNCs. Values are mean ± SD for three independent replicates. Data analyzed using two-tailed Student’s t-test (A) or one-way analysis of variance (B-D). |
|
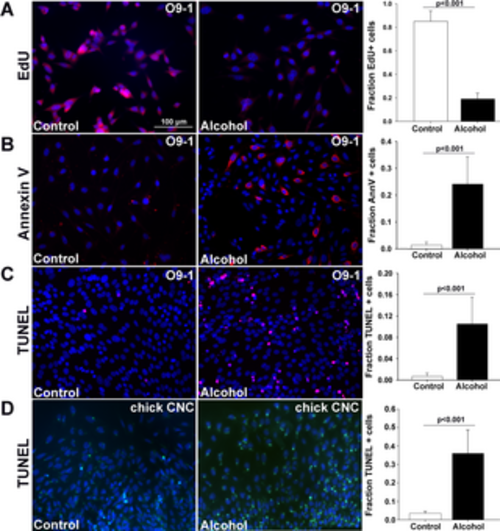
Alcohol causes apoptosis in the O9-1 cells and primary chick CNCs. Cells were exposed to alcohol at the EC50 (80mM for O9-1 cells, 52mM for primary CNCs) for 2hr and assessed at times thereafter. (A) Alcohol reduced the percentage of EdU+ proliferating cells in O9-1 cells 12hr after exposure. (B) Alcohol increased the percentage of Annexin-V+ (red) cells in O9-1 cells 6hr after exposure. (C) Alcohol increased the percentage of TUNEL+ (red) O9-1 cells 18hr after exposure. (D) Alcohol increased the percentage of TUNEL+ (green) primary chick CNCs at 18hr after exposure. Values are the mean ± SEM of 5–8 independent experiments. Data were analyzed using two-tailed Student’s t-test. |
|
Alcohol causes p53/MDM2-dependent apoptosis in the mouse CNC line O9-1. (A) Immunostain reveals that p53 protein (red) is detected predominantly in the cytosol of untreated O9-1 cells, and this protein becomes stabilized within the nuclei (blue) 6hr after alcohol exposure. Right panel, alcohol increases the percentage of p53+ nuclei. (B) Alcohol exposure causes a six-fold rise in p53 protein content in O9-1 cells at 6hr following exposure. (C) Transfection of dominant-negative p53 (p53-DN) into O9-1 cells prevents the alcohol-induced apoptosis as detected using TUNEL (red signal) and does not affect the survival of unexposed cells, whereas transfection with the parent pCS2 vector does not affect cell survival. Right panel, alcohol increases the percentage of TUNEL+ cells and this is prevented by overexpression of dominant-negative p53 (+p53DN) but not by transfection with the empty vector (-p53DN). (D) Overexpression of MDM2 prevents the alcohol-induced apoptosis as detected by TUNEL (red) but does not affect the survival of control cells, compared with cells transfected with the empty vector pCS2. Right panel, alcohol increases the percentage of TUNEL+ O9-1 cells and this is normalized by MDM2. All values are mean ± SEM of 2 independent experiments as detailed in Methods. Data were analyzed using equal variance t-test (A, B) or one-way analysis of variance with Holm-Sidak multiple comparisons (C, D). |
|
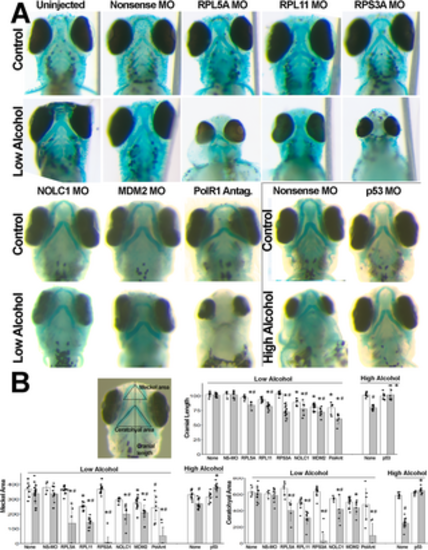
Haploinsufficiency in ribosome biogenesis-related genes heightens vulnerability to alcohol-induced craniofacial deficits in zebrafish embryos. (A) Zebrafish embryos injected with nonsense MO, or MOs against RPL5A, RPL11, RPS3A, NOLC1, p53, or MDM2 had no or modest deficits in the size or shape of cranial cartilage elements as compared with no-MO controls. Low-dose alcohol-only also did not worsen cranial development. The combination of low-dose alcohol and morpholinos directed against genes that promote ribosome biogenesis (rpl5a, rpl11, rps3a, nolc1) and p53/MDM2 signaling (mdm2) synergized to worsen cranial development and, in some instances, ablated cranial cartilage. Similarly, low-doses of the RNA polymerase I inhibitor CX5461 modestly affected cranial cartilage and synergized with low-dose alcohol to eliminate facial cartilage. Conversely, high-dose alcohol (boxed panels) caused craniofacial reductions that were prevented by morpholinos directed against p53. All views are ventral at 4 dpf with equivalent magnification. (B) Depiction of morphometry measurements, and quantification of cranial length, Meckel area, and ceratohyal area. Units are arbitrary and normalized to the mean of the uninjected controls. Open bars, no-alcohol; shaded bars, alcohol at the low dose or high dose as indicated. All values are mean ± SD with 4–14 embryos per treatment as detailed in Methods. Data analyzed using two-way analysis of variance followed by post hoc analysis using Holm-Sidak multiple comparisons (morpholino, compound) or within-group comparisons (alcohol vs. no-alcohol). * Different at p≤0.001 from its exposure-matched control for either nonsense-MO vs. gene-morpholino or no-compound vs. compound-treated. # Different at p≤0.001 from its treatment-matched control, Alcohol vs. no-Alcohol. |