- Title
-
Microbe transmission from pet shop to lab-reared zebrafish reveals a pathogenic birnavirus
- Authors
- Rice, M.C., Janik, A.J., Elde, N.C., Gagnon, J.A., Balla, K.M.
- Source
- Full text @ PLoS Biol.
|
Metatranscriptomic survey of microbes associated with zebrafish from laboratory and pet trade sources. . (A) Schematic representation of a newly discovered birnavirus associated with zebrafish in this study. Both segments of the linear dsRNA genome are depicted with boxes denoting relative positions of ORFs based on protein domain conservation. Numbers beneath segments indicate nucleotide lengths of each prospective 5′ UTR, coding sequence, and 3′ UTR. (B) Maximum likelihood phylogeny of viruses in the family Birnaviridae. The black dot highlights the novel zebrafish-associated virus schematized in (A), which we name RMBV. (C) Maximum likelihood phylogeny of RdRp protein sequences from viruses in the order Picornavirales. Black dots highlight the zebrafish-associated viruses that were detected (ZfPV) or newly discovered in this study. Trees in (A and C) are midpoint rooted. Scales are in amino acid substitutions per site. Branches are colored based on the known or inferred host group. (D) Taxonomic classification and quantification of host and microbe transcripts identified in intestinal tissues of zebrafish from laboratory and pet trade sources. Columns show estimated abundances in tissue samples from independent individuals grouped by source. RdRp, RNA-dependent RNA polymerase; RMBV, Rocky Mountain birnavirus; ZfPV, zebrafish picornavirus. |
|
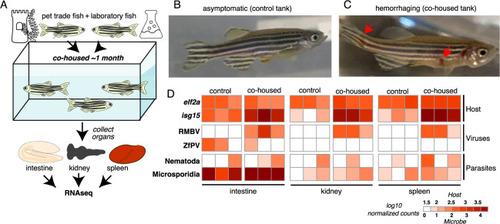
Overt disease observed in laboratory zebrafish cohoused with zebrafish from the pet trade is associated with RMBV infection. (A) Diagram of cohousing experiment. Tanks were seeded with adult zebrafish from the laboratory (control) or zebrafish from the laboratory and the pet trade (cohoused). (B) A representative individual from the control tank. (C) A representative laboratory zebrafish 1 month after cohousing with zebrafish from the pet trade. Red arrows highlight necrotic lesions. (D) Transmission of microbes from pet trade to cohoused laboratory zebrafish inferred from host and microbe transcript abundances. Three laboratory fish were sampled from each tank. Samples are grouped by tank and tissue. Columns show estimated transcript abundances in tissue samples from individual laboratory zebrafish. RMBV, Rocky Mountain birnavirus. |
|
Naturally transmitted RMBV infections induce systemic inflammatory antiviral responses in laboratory zebrafish. (A) Differential gene expression in laboratory zebrafish infected by RMBV compared to uninfected laboratory zebrafish. Average log2 fold change expression is plotted for each gene in a comparison of 3 RMBV-infected and 3 uninfected samples for each tissue. All comparisons had adjusted |