- Title
-
Suppression of Contraction Raises Calcium Ion Levels in the Heart of Zebrafish Larvae
- Authors
- Martinez-Sielva, A., Vicente, M., Salgado-Almario, J., Garcia-Blazquez, A., Domingo, B., Llopis, J.
- Source
- Full text @ Biosensors (Basel)
|
Morphological and functional alterations in 3 dpf |
|
Increased Ca2+ levels, Ca2+ transient amplitude, and bradycardia in 3 dpf larvae injected with |
|
Increased Ca2+ levels, Ca2+ transient amplitude, and bradycardia in 3 dpf |
|
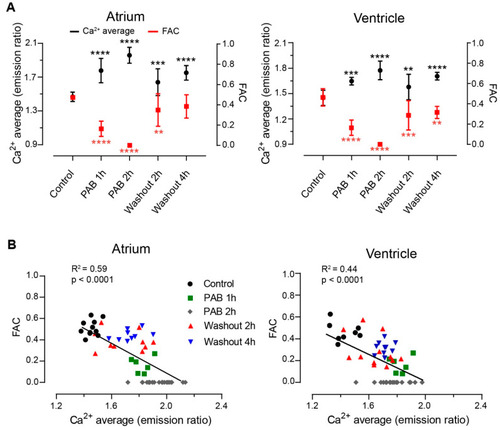
Suppression of heart contraction with |
|
Incubation and washout of |