- Title
-
Assessment of the FRET-based Teen sensor to monitor ERK activation changes preceding morphological defects in a RASopathy zebrafish model and phenotypic rescue by MEK inhibitor
- Authors
- Fasano, G., Petrini, S., Bonavolontà, V., Paradisi, G., Pedalino, C., Tartaglia, M., Lauri, A.
- Source
- Full text @ Mol. Med.
|
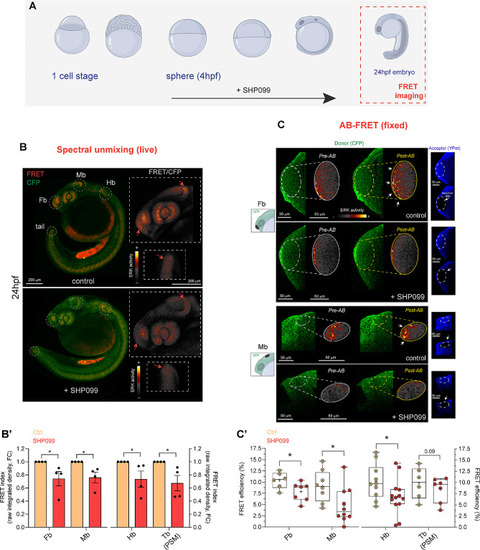
Decreased ERK activity in live and fixed Teen embryos upon prolonged SHP099 exposure is captured by spectral unmixing- and AB-FRET. ( |
|
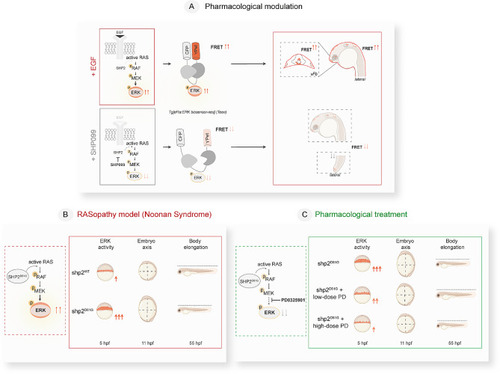
Schematics of the study design and outcome summarizing the main steps and outcomes of pharmacologically- and genetically induced RAS-MAPK pathway modulation assessed in |
|
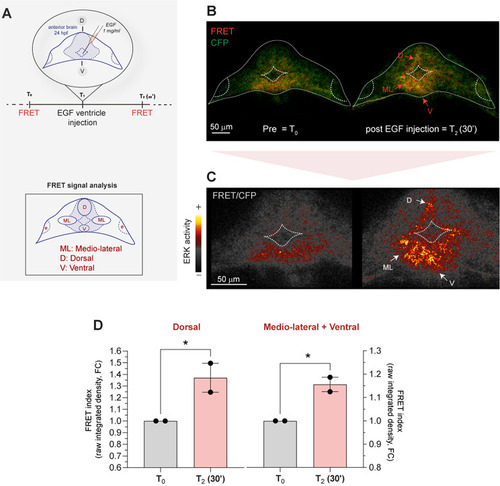
Increase in ERK activity is observed by spectral unmixing-FRET in the forebrain of live |
|
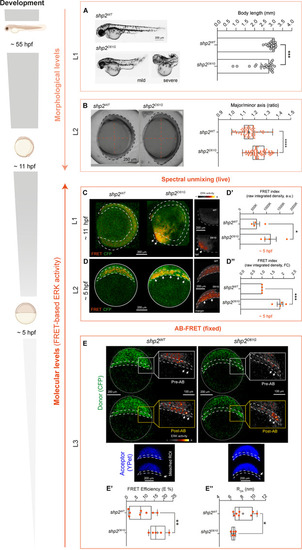
Increased ERK signal measured by spectral unmixing and AB-FRET in Shp2D61G zebrafish mutants showing morphological defects. ( |
|
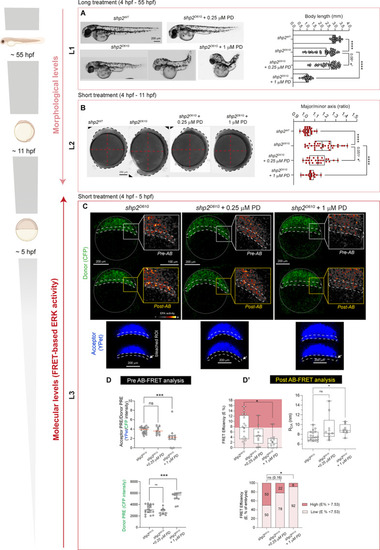
Reduced ERK activity measured by spectral unmixing and AB-FRET in zebrafish Shp2D61G NS-causing mutants exhibiting morphological defects upon low- and high-dose MEK inhibitor treatment. ( |
|
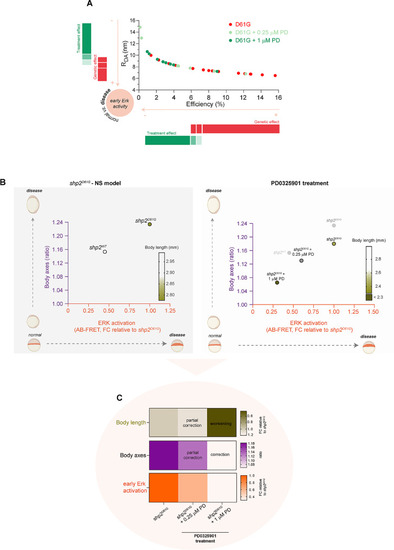
Molecular and morphological effect of the treatment with MEK inhibitor PD0325901 on zebrafish embryos overexpressing Shp2D61G and showing developmental features modeling NS. ( |