- Title
-
Bi-allelic variants in SNF8 cause a disease spectrum ranging from severe developmental and epileptic encephalopathy to syndromic optic atrophy
- Authors
- Brugger, M., Lauri, A., Zhen, Y., Gramegna, L.L., Zott, B., Sekulić, N., Fasano, G., Kopajtich, R., Cordeddu, V., Radio, F.C., Mancini, C., Pizzi, S., Paradisi, G., Zanni, G., Vasco, G., Carrozzo, R., Palombo, F., Tonon, C., Lodi, R., La Morgia, C., Arelin, M., Blechschmidt, C., Finck, T., Sørensen, V., Kreiser, K., Strobl-Wildemann, G., Daum, H., Michaelson-Cohen, R., Ziccardi, L., Zampino, G., Prokisch, H., Abou Jamra, R., Fiorini, C., Arzberger, T., Winkelmann, J., Caporali, L., Carelli, V., Stenmark, H., Tartaglia, M., Wagner, M.
- Source
- Full text @ Am. J. Hum. Genet.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
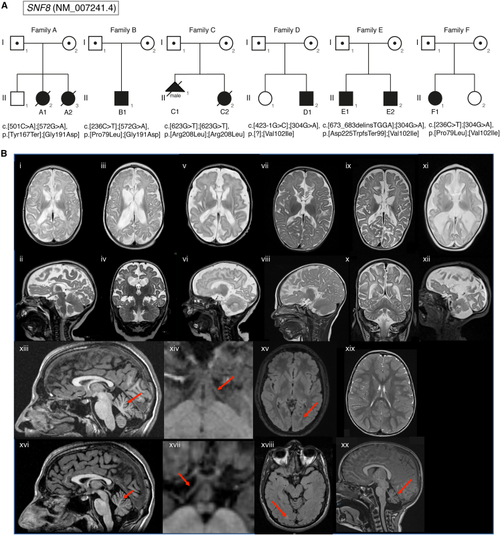
Pedigrees of the families with bi-allelic (A) Pedigrees of the families A–F. Variants identified in the individuals are depicted underneath the corresponding symbol. In family A, the healthy male sibling (II-1) of individuals A1 and A2 was not compound heterozygous for the (B) Brain MRI scans of the affected individuals with pathogenic variants of |
|
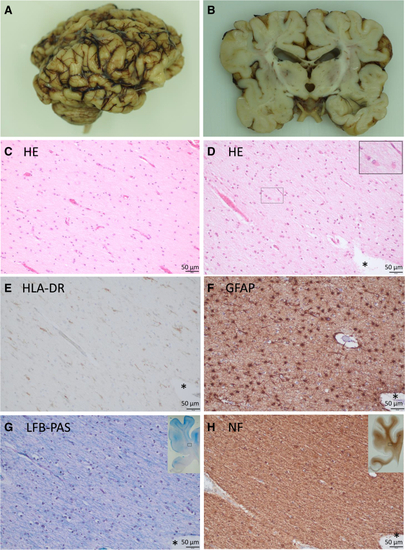
Macroscopic and microscopic alterations in individual A2 deceased at the age of 3 months (A) Lateral view on the formalin-fixed autopsy brain. (B) Coronally cut slice of the formalin-fixed brain at the level of thalamus/hippocampus showing a massive reduction of the subcortical white matter and a severe atrophy of the corpus callosum. The gyri appear coarse. (C–H) Frontal subcortical white matter at level of anterior cingulate gyrus; (C) age-matched control. (D–H) Consecutive sections in individual A2. (C and D) Hematoxylin-eosin (HE) stains. Compared to the age-matched control case (C), the astrocytes of individual A2 (D) are reactively altered showing enlarged eosinophilic cell bodies (inset displays zoomed marked area). The number of astrocytes is also increased in individual A2. (E) With an antibody against human leukocyte antigen-DR isotype (HLA-DR), numerous activated microglial cells (brown color) are visualized as a sign of a resorptive process. (F) The immunohistochemical detection of glial fibrillary acidic protein (GFAP; brown color) illustrates the large number of reactively changed astrocytes. (G) Myelin is dramatically reduced (blue color) in a luxol fast blue-periodic acid Schiff (LFB-PAS) stain, while in (H) the axons are relatively well preserved as demonstrated by immunohistochemistry for neurofilament (NF; brown color). Insets in (G) and (H) show overviews of the slides, rectangles mark zoomed areas. ∗Highlights the same blood vessel in consecutive tissue sections. Scale bars in (C)–(H) correspond to 50 μm. |
|
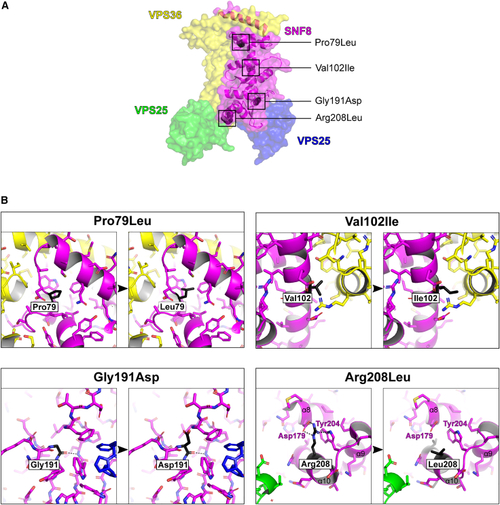
Representation of the 3D structure of SNF8 with the observed missense variants (A) The surface rendering of human ESCRT II complex (PDB: (B) Magnified view of the residues affected by the reported missense variants. The affected residues are shown in black and labeled accordingly. Hydrogen bonds involving affected residues are depicted as dashed black lines. The variants p.Pro79Leu and p.Gly191Asp introduce larger residues that lead to steric clashes with surrounding residues and likely affect the conformation and stability of SNF8. In contrast, the p.Arg208Leu variant replaces the long and charged arginine with a short and hydrophobic leucine, resulting in a loss of interaction with aspartic acid 179 and tryptophan 204, thus most likely also affecting the conformation and stability of SNF8. The most conservative amino acid substitution with the least impact is p.Val102Ile, in which the small hydrophobic valine is replaced by a slightly larger (one atom) hydrophobic isoleucine. |
|
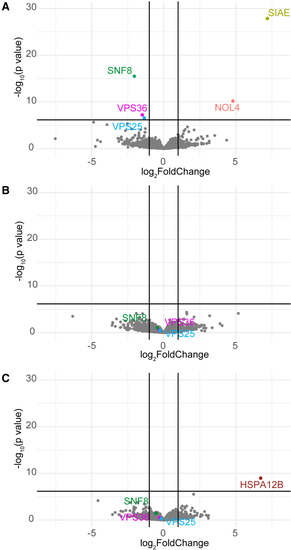
Effects of bi-allelic variants in Volcano plot of the proteomics analysis performed on cultured fibroblasts from individual A2 (A), D1 (B), and E1 (C). SNF8, VPS25, VPS36, and proteins with statistically different levels are highlighted in color. Vertical black lines indicate log2fold changes of −1 and 1. Horizontal black lines depict significance level of p = 7.14 × 10−6 (Bonferroni correction for 7,000 hypotheses representing the number of proteins identified). Note the significant reduced protein levels of ESCRT II complex subunits SNF8, VPS25, and VPS36 for individual A2. |
|
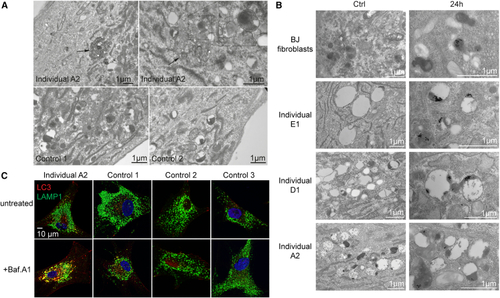
Bi-allelic variants in (A) Fibroblasts derived from individual A2 (top) or control individuals (bottom) were analyzed by transmission electron microscopy. Arrows indicate large vesicular structures that contain cytoplasmic content, consistent with being autolysosomes. Scale bars: 1 μm. (B) Fibroblasts were incubated with BSA-gold (10 nm) for 24 h and washed and then the gold was chased overnight to accumulate in lysosomal compartments. Cells were then studied by transmission electron microscopy. Images of untreated fibroblasts are found on the left (Ctrl) whereas images on the right side (24 h) represent fibroblasts stained with BSA-gold. In BJ control fibroblasts, most of the gold tracer is found in uniformly dense lysosomes. In patient-derived cells, the gold is found in the dense part of bigger lysosomal compartments, which mostly consist of an electron-lucent lumen. This phenotype is similar for the various patient-derived fibroblasts. Scale bars: 1 μm. (C) Fibroblasts derived from individual A2 or control individuals were left untreated of incubated with Bafilomycin A1 and then fixed and processed for immunofluorescence confocal microscopy with antibodies against LC3 and LAMP1. Co-localization between the two markers after Bafilomycin A1 treatment is indicative of autolysosomes. Scale bar: 10 μm. |
|
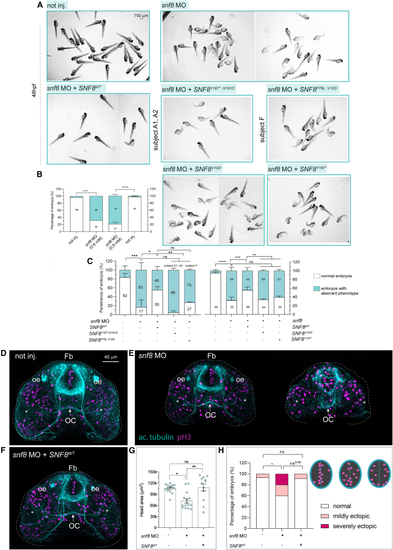
Snf8 loss of function in zebrafish leads to developmental defects that are rescued by expression of the wild-type (A) Representative bright-field pictures of fish not injected (not inj.), zebrafish injected with (B and C) Quantification of the percentage of fish showing normal (white) or aberrant (cyan) development upon injection of different (D–F) Representative confocal maximum intensity projections of fish fluorescently stained with antibodies against acetylated tubulin (cyan) and pH3 (magenta) from not injected (not inj.) (D), mild and severe cases of fish injected with (G) Quantification of the overall brain area from the confocal z-projections. (H) Number of embryos showing “normal” or “mildly or severely ectopic” proliferative cells (pH3+) within the anterior ventral forebrain. n = 15 (not inj. and Number of replicates in (B), one and two (left and right graphs, respectively). In (C) (left), number of replicates: three for not inj, PHENOTYPE:
|
|
Zebrafish embryos injected with (A) Representative confocal maximum intensity projections of fish fluorescently stained with the antibody against acetylated tubulin (cyan) from not injected (not inj., up), mild, moderate, and severe cases of fish injected with (B) Quantification of the percentage of fish showing normal (white) or mild, moderate, or severely aberrant (gradients of cyan) ON phenotypes as described in the main text. n = 15 (not inj.), 15 ( (C and D) Quantification of the optic nerve length, measured as ON extension between two eyes and thickness, measured on both side of ON. In (C), n = 15 (not inj.), 15 ( (E) Quantification of the dimension of the angle formed by the optic chiasm (OC) in the midline. n = 15 (not inj.), 9 ( PHENOTYPE:
|