- Title
-
Embryonic ethanol exposure and optogenetic activation of hypocretin neurons stimulate similar behaviors early in life associated with later alcohol consumption
- Authors
- Collier, A.D., Yasmin, N., Karatayev, O., Abdulai, A.R., Yu, B., Fam, M., Campbell, S., Leibowitz, S.F.
- Source
- Full text @ Sci. Rep.
|
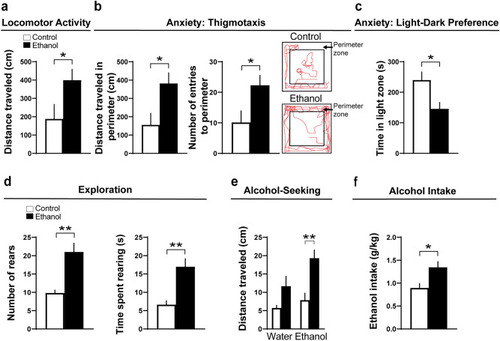
Effects of embryonic ethanol exposure (2 g/kg/day, from E10–E15) compared to control on alcohol-related behaviors, including locomotor activity, anxiety, exploration and alcohol-seeking in pre-weanling female rats and voluntary consumption of alcohol in adolescent female rats. ( |
|
Effects of embryonic ethanol exposure (0.5% v/v, from 22–24 hpf) compared to control on alcohol-related behaviors, including locomotor activity and anxiety-like behaviors, in larval zebrafish (6 dpf). ( |
|
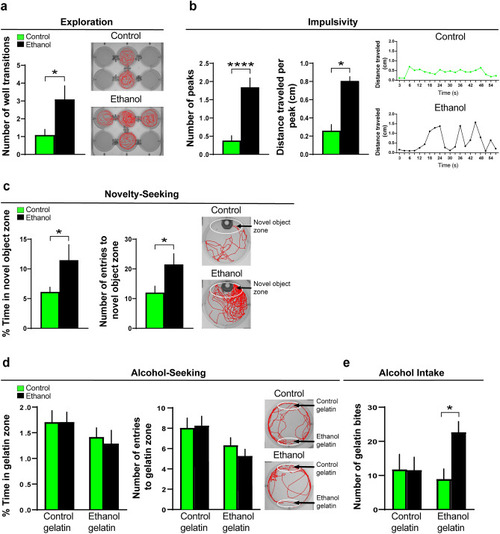
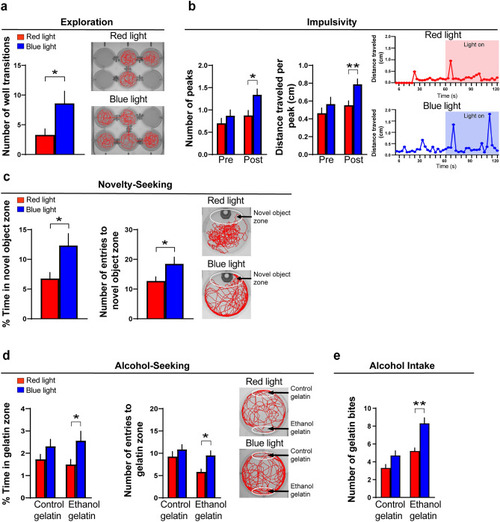
Effects of embryonic ethanol exposure (0.5% v/v, from 22 to 24 hpf) compared to control on alcohol-related behaviors, including exploration, impulsivity, novelty-seeking and alcohol-seeking in 6 dpf zebrafish and voluntary intake of ethanol-gelatin in juvenile zebrafish (30 dpf). ( |
|
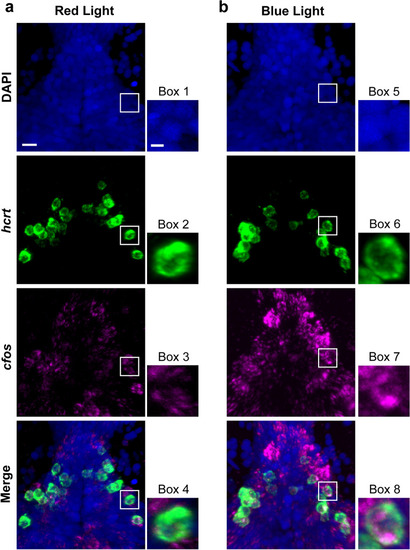
Representative photomicrographs illustrating increased colocalization of transcripts of the immediate early gene cfos, a marker of neuronal activation, within |
|
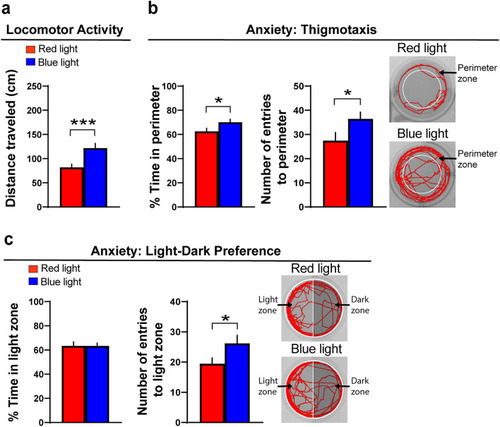
Effects of optogenetic activation of Hcrt neurons on alcohol-related behaviors, including locomotor activity and anxiety, in transgenic |
|
Effects of optogenetic activation of Hcrt neurons in transgenic |