- Title
-
Trpv4-mediated apoptosis of Leydig cells induced by high temperature regulates sperm development and motility in zebrafish
- Authors
- Yamamoto, Y., Hishikawa, D., Ono, F.
- Source
- Full text @ Commun Biol
|
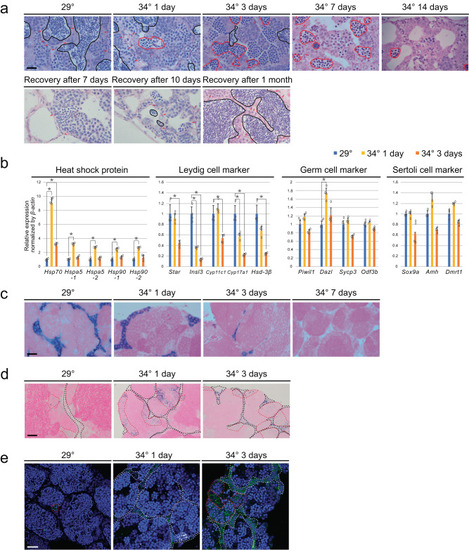
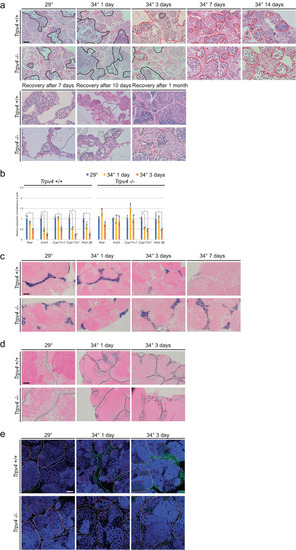
High-temperature treatment led to significant apoptosis of Leydig cells. |
|
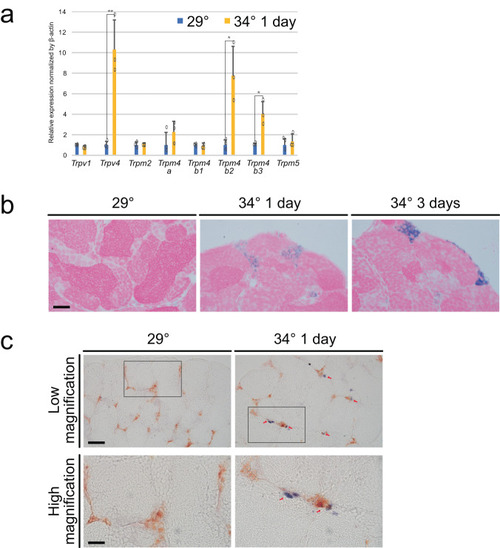
Higher temperature led to upregulated expression of |
|
Leydig cells in |
|
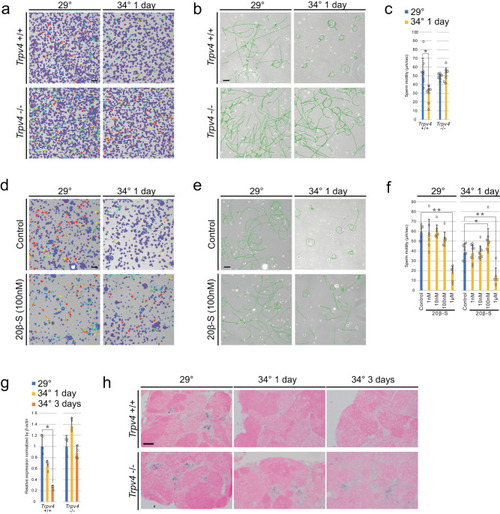
Higher temperature impaired sperm motility in |
|
Sperm matured at high temperature showed an abnormality. |