- Title
-
Osteomodulin downregulation is associated with osteoarthritis development
- Authors
- Zappia, J., Tong, Q., Van der Cruyssen, R., Cornelis, F.M.F., Lambert, C., Pinto Coelho, T., Grisart, J., Kague, E., Lories, R.J., Muller, M., Elewaut, D., Hammond, C.L., Sanchez, C., Henrotin, Y.
- Source
- Full text @ Bone Res
|
Localization of OMD in the murine knee joint. |
|
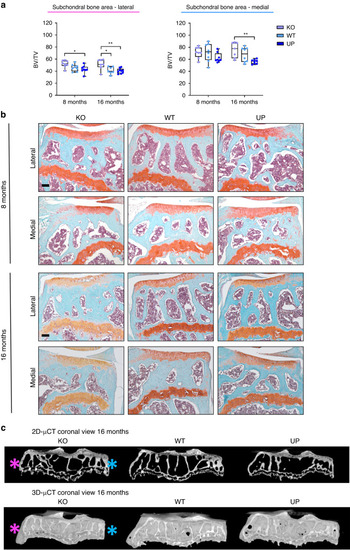
Histomorphometry of the cartilage was performed with QuPath at 4, 8, and 16 months. Knee joints of male mice were stained with Toluidine blue and areas corresponding to the total cartilage, the calcified cartilage, and the growth plate were measured for the medial tibial plateau and the lateral tibial plateau. |
|
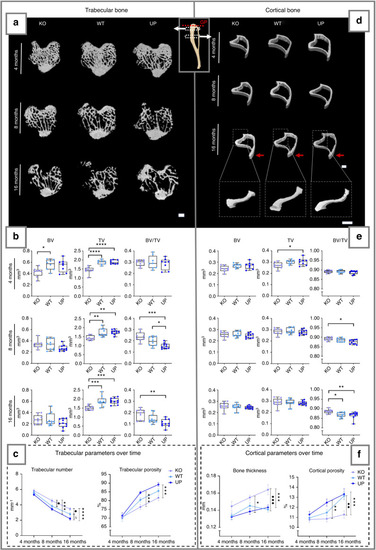
µCT analysis of the metaphysis of the tibia of the male mice at 4, 8 and 16 months. The trabecular bone (left) and the cortical bone (right) were analyzed separately. Regions measured for the trabecular bone and cortical bone are illustrated on the schematic tibia with the growth plate (GP), marked with a red dotted line, used as a reference for their selection. |
|
Effect of |
|
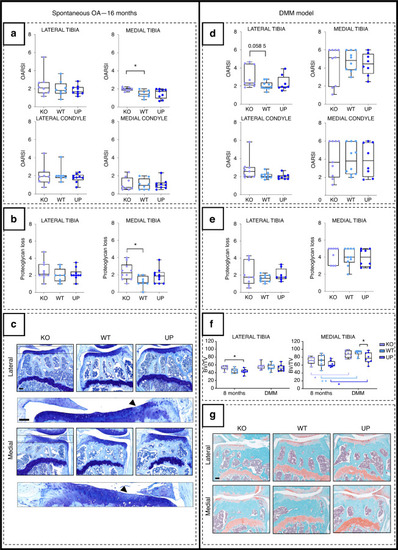
Analysis of the development of OA lesions in the different genotypes after spontaneously occurring with age (left) or after the DMM (right). The spontaneous OA lesions were considered in the 16-month-old male mice and the DMM was performed on 16-week-old male mice and they were stopped at 28 weeks. |
|
Analysis of the gait of 4-, 8-, and 16-month-old male mice with the CatWalk XT. The intensity corresponds to the mean intensity at the maximum paw contact normalized with the mean of the maximum contact paw area, the speed, and the weight of the mouse. At 4 months: |
|
The mutant deficient for PHENOTYPE:
|
|
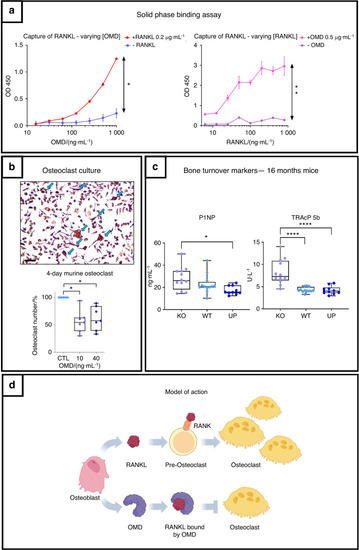
OMD inhibits osteoclastogenesis through its direct interaction with RANKL and balances bone remodeling. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |