- Title
-
Loss of growth differentiation factor 9 causes an arrest of early folliculogenesis in zebrafish-A novel insight into its action mechanism
- Authors
- Chen, W., Zhai, Y., Zhu, B., Wu, K., Fan, Y., Zhou, X., Liu, L., Ge, W.
- Source
- Full text @ PLoS Genet.
|
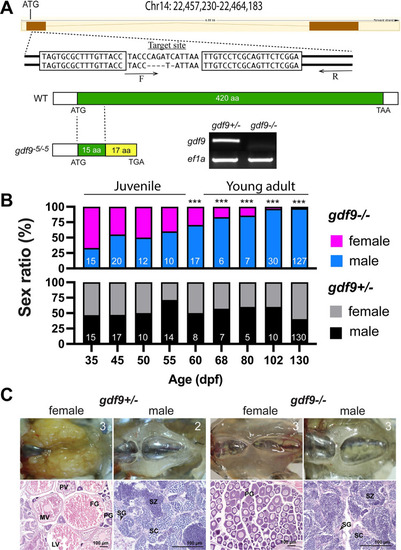
Disruption of gdf9 gene in zebrafish and evidence for its roles in gonadal differentiation and gonadal development.
(A) Generation of gdf9 mutant by TALEN. The target site on chromosome 14 for left and right TALE proteins are boxed. A mutant with 5-bp deletion was obtained with a premature stop codon (TGA), which generated a truncated protein with 32 amino acids (15 from the Gdf9 precursor). RT-PCR analysis on mRNA from the ovary using a WT-specific primer (F) showed a positive signal in the control ovary (gdf9+/-), but not in the mutant (gdf9-/-). (B) Sex ratios during and after gonadal differentiation in gdf9 mutant fish. The offspring from one crossing (gdf9+/- female X gdf9-/- male) were sampled at different times from 35 to 130 dpf for genotyping and sex identification by histology. The sex ratios in the post-differentiation period from 35 to 55 dpf remained relatively constant and comparable to those in the control fish. However, the ratio changed gradually but significantly afterwards towards all males. The number of fish examined is shown at the bottom of each column. *** P < 0.001 (X2 test). (C) The ovary and testis in the control (gdf9+/-) and mutant (gdf9-/-) at 90 dpf. The control females had reached sexual maturity with all stages of follicles present in the ovary (PG, primary growth; PV, pre-vitellogenic; MV, mid-vitellogenic; LV, late vitellogenic; and FG, full-grown) while the mutant ovary contained PG follicles only. The testis development and spermatogenesis showed no difference between mutant and control males. SG, spermatogonia; SC, spermatocytes; SZ, spermatozoa. The number in each photo indicates the number of fish examined with the same phenotype. PHENOTYPE:
|
|
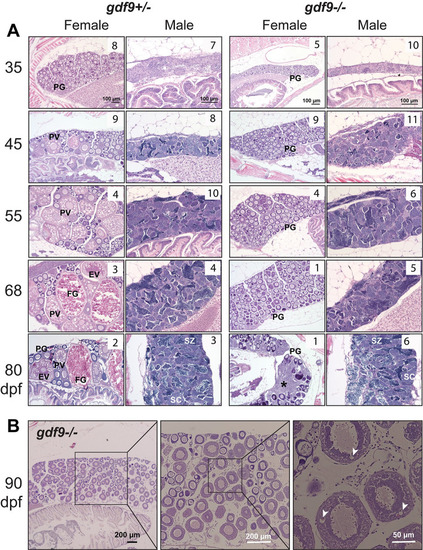
Gonadal development from sex differentiation to maturity in gdf9 mutant.
(A) Temporal development of the ovary and testis in the control (gdf9+/-) and mutant (gdf9-/-) from gonadal differentiation (35 dpf) to puberty onset (45 dpf) and sex maturity (80 dpf). The mutant females started to differ from the control at 45 dpf when the first cohort of PV follicles with cortical alveoli emerged in the control ovary, marking the onset of puberty. No PV follicles were observed in the mutant ovary, and the follicles remained arrested at PG stage during the entire examination period. At 80 dpf, the mutant females were undergoing sex reversal to males with remnant follicles and infiltrating stromal and testicular tissues (asterisk). The testis and spermatogenesis were normal in the mutant males compared to the control. The number in each photo indicates the number of fish examined with the same phenotype. (B) Very few gdf9 mutant females could cross the PG-PV transition and their follicles showed rudiment cortical alveoli (arrowhead). PG, primary growth; PV, pre-vitellogenic; EV, early vitellogenic; FG, full-grown; SC, spermatocytes; SZ, spermatozoa. PHENOTYPE:
|
|
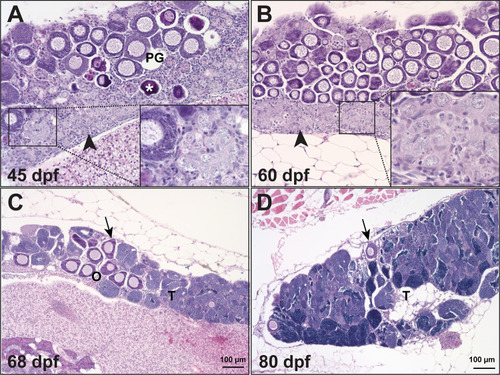
Temporal process of sex reversal from females to males.
Sex reversal in gdf9 mutant started around 45 dpf when the follicles were undergoing degeneration (asterisk) while stromal tissue with early spermatogenic cells (inset) was infiltrating the ovarian tissue, which normally started from the ventral side of the ovary (arrowhead). The number of PG follicles (arrow) decreased progressively during the sampling period (45–80 dpf) with increasing amount of testicular tissue (T). |
|
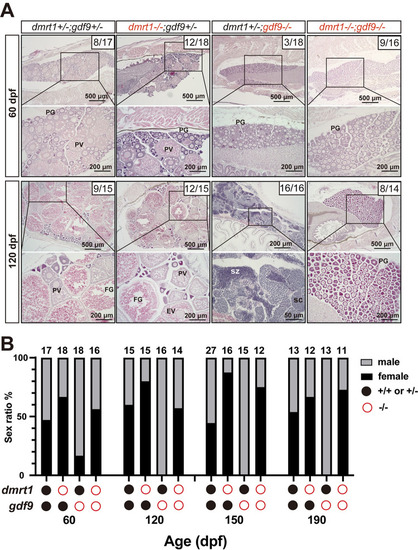
Prevention of sex reversal of gdf9 mutant by dmrt1 mutation.
(A) Gonadal histology at 60 and 120 dpf. Ovary could form in the control (dmrt1+/-;gdf9+/-), single mutants (dmrt1-/- and gdf9-/-) and double mutant (dmrt1-/-;gdf9-/-) as seen at 60 dpf. The gdf9-/- single mutant became all males at 120 dpf (16/16 fish examined in total) whereas most individuals of the double mutant were females (8/14 examined), similar to that in the control (9/15). The numbers shown in the photos indicate the total number of fish examined (lower) and the number of female fish exhibiting similar phenotype to that shown (upper). (B) Change of sex ratio during 60–190 dpf. The offspring from one crossing (dmrt1+/-;gdf9+/- female X dmrt1+/-;gdf9+/- male) were sampled at different times from 60 to 190 dpf for genotyping and sex identification by histology. The gdf9-/- fish showed a male-biased sex ratio at 60 dpf, and it became all-male from 120 dpf due to sex reversal. Double mutation with dmrt1 rescued the sex ratio to the control level with even higher female ratios at 150 and 190 dpf. PG, primary growth; PV, pre-vitellogenic; FG, full-grown; SC, spermatocytes; SZ, spermatozoa. PHENOTYPE:
|
|
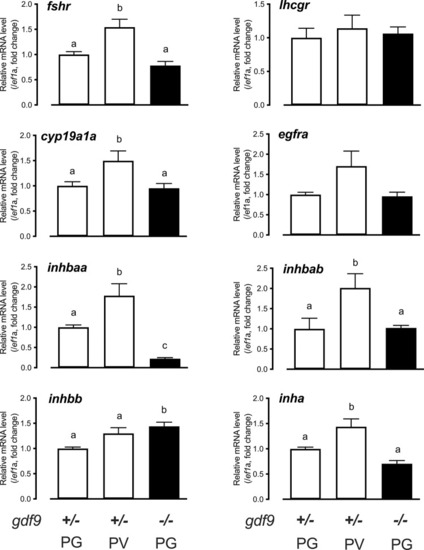
Gene expression in the control and mutant follicles at 45 dpf.
Follicles were isolated from the control (gdf9+/-) (PG and PV) and mutant (gdf9-/-) (PG only) for total RNA extraction and real-time qPCR analysis. The data were normalized to the house-keeping gene ef1a and then expressed as the fold change over the control PG. Different letters indicate statistical significance (P < 0.05; n = 3–4 biological replicates). fshr, FSH receptor; lhcgr, LH receptor; cyp19a1a, ovarian aromatase; egfra, EGF receptor a; inhbaa, activin βAa; inhbab, activin βAb; inhbb, activin βB; inha, inhibin α. EXPRESSION / LABELING:
|
|
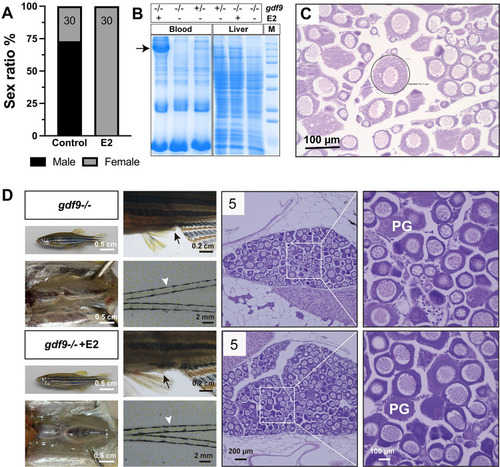
Estrogen treatment of gdf9 mutant.
(A-C) Treatment with estradiol (E2) by water-borne exposure. The mutant fish (30/group) were treated from 40 to 60 dpf with E2 (10 nM). E2 treatment resulted in all-female phenotype in contrast to untreated group which showed a male-biased sex ratio (A). Electrophoresis of the sera (1 μl/lane) from treated mutant females showed mass production of vitellogenin protein (arrow); in contrast, there was no vitellogenin in the sera of untreated mutant fish (-/-). The control fish (+/-) without E2 treatment showed a weak band at the location of vitellogenin. A similar band could also be seen in the liver extract (10 μg/lane) in response to E2 treatment. Each lane represents one representative individual, and the serum and liver samples were obtained from the same individual for each genotype and treatment combination (B). Histology of the ovary from E2-treated fish showed arrest of follicles at PG stage with a few follicles (circled) showing rudiment cortical alveoli in some individuals (C). (D) Treatment with E2 by oral administration (feeding). The mutant fish were fed with E2-containing diet (20 μg/g) for 17 days (45–62 dpf) followed by examination for secondary sex characteristics (genital papilla in females, arrow; breeding tubercles in males, arrowhead) and gonadal histology. The treated group showed higher female ratio (44% females, 8/18) compared to the control group (21% females, 3/14). Females from both groups showed normal genital papilla with no breeding tubercles and arrested PG follicles in the ovary. The number in each histological photo indicates the number of fish examined with the same phenotype. PG, primary growth. PHENOTYPE:
|
|
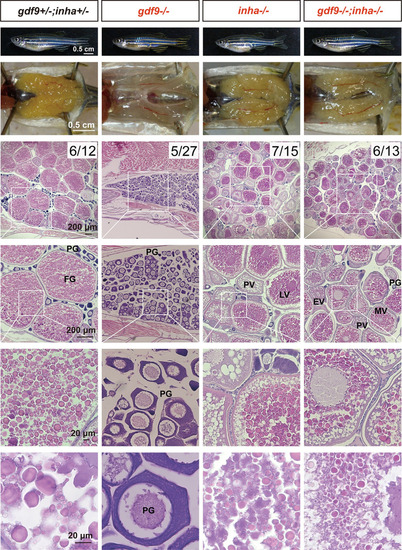
Rescue of follicle blockade in gdf9 mutant by mutation of inhibin α (inha-/-).
The follicles in gdf9 mutant (gdf9-/-) were arrested at PG stage at 90 dpf, and double mutation of gdf9 and inha (gdf9-/-;inha-/-) resumed follicle activation or PG-PV transition and vitellogenic growth. The numbers shown in the photos indicate the total number of fish examined (lower) and the number of female fish exhibiting similar phenotype to that shown (upper). PG, primary growth; PV, pre-vitellogenic; EV, early vitellogenic; MV, mid-vitellogenic; LV, late vitellogenic; FG, full-grown. PHENOTYPE:
|
|
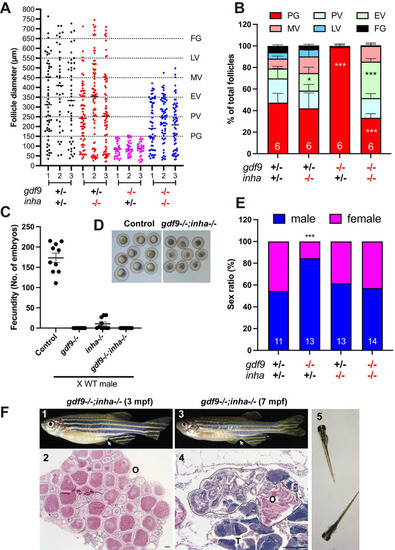
Phenotype analysis of single (gdf9-/- and inha-/-) and double mutants (gdf9-/-;inha-/-).
(A and B) Follicle composition in different genotypes at 90 dpf. The follicles in gdf9-/- were arrested at PG stage. Although the PG-PV transition and yolk accumulation resumed in the double mutant (gdf9-/-;inha-/-), the vitellogenic growth could not proceed to the FG stage, and the follicles were arrested at the MV-LV transition. * P < 0.05; *** P < 0.001 (X2 test, n = 6). (C) Fertility test on females of single (gdf9-/- and inha-/-) and double mutants (gdf9-/-;inha-/-) at 90 dpf. Five mutant and control females were tested 10 times consecutively every other day by natural pair breeding with WT males (n = 5/test). The test was conducted over about 20 days. In each test, the number of fertilized eggs or embryos was counted for each female and the data point represents the average of the five fish tested. (D) Eggs spawned by the control (gdf9+/-;inha+/-) and double mutant (gdf9-/-;inha-/-). The eggs from the double mutant were smaller than those from the control. (E) Prevention of sex reversal in gdf9-/- by double mutation with inha-/- at 90 dpf. The gdf9-/- fish showed a male-biased sex ratio (82% males), and this was corrected in the double mutant (47% males). *** P < 0.001 (X2 test, n = 11–14). (F) Sex reversal of the gdf9 and inha double mutant (gdf9-/-;inha-/-) at 7 mpf. (1) Double mutant female at 3 mpf with female-specific genital papilla (arrow); (2) Ovary of 3-mpf double mutant; (3) Sex reversed intersexual fish at 7 mpf with regressed genital papilla (arrow); (4) Ovotestis of 7-mpf double mutant. The follicles in the ovarian tissue (O) were degenerating (circled by dotted line) and the testicular tissue (T) became dominant in the gonads. (5) The offspring from sex reversed double mutant males. PHENOTYPE:
|
|
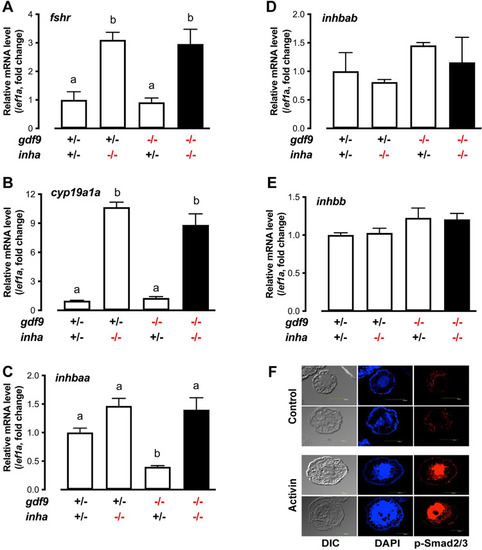
Expression of fshr, cyp19a1a and activins (inhbaa, inhbab and inhbb) in PG follicles from single (gdf9-/- and inha-/-) and double mutant (gdf9-/-;inha-/-).
(A-E) The PG follicles were isolated from the fish of different genotypes at 43 dpf, shortly before puberty onset or PG-PV transition occurs in the control fish (normally around 45 dpf). The expression of inhbaa was significantly reduced in gdf9-/- mutant; however, it was reversed by inha mutation in the double mutant together with fshr and cyp19a1a, but not inhbab and inhbb. Different letters indicate statistical significance (P < 0.05, n = 3 biological replicates). (F) Stimulation of Smad2/3 phosphorylation (p-Smad2/3) in the PV oocytes by activin in vitro. The PV follicles were isolated and treated with recombinant goldfish activin B (5 U/ml) for 2 h followed by immunofluorescent staining for p-Smad2/3. EXPRESSION / LABELING:
PHENOTYPE:
|
|
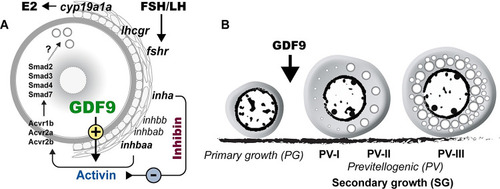
Hypothetical model on roles of oocyte-derived GDF9 (gdf9) in controlling zebrafish folliculogenesis.
(A) Intrafollicular distribution of GDF9 and other important genes (fshr, lhcgr, cyp19a1a, inhbaa/ab, inhbb, inha, acvr1b, acvr2a/b and smad2/3/3/7). (B) GDF9 from the oocyte plays an important gating role in controlling zebrafish follicle activation or PG-PV transition and therefore puberty onset. PV-I, early PV with one single layer of small cortical alveoli; PV-II, mid-PV with one single layer of large cortical alveoli; PV-III, late PV with multiple layers of cortical alveoli. |