- Title
-
Evaluation of Cisplatin-Induced Pathology in the Larval Zebrafish Lateral Line
- Authors
- Lee, D.S., Schrader, A., Bell, E., Warchol, M.E., Sheets, L.
- Source
- Full text @ Int. J. Mol. Sci.
|
Dose- and time- response relationships of lateral line hair cell loss following 2 h exposure to cisplatin. ( PHENOTYPE:
|
|
Macrophages respond to cisplatin-induced injury and phagocytose dying hair cells. ( PHENOTYPE:
|
|
Depleting macrophages does not affect cisplatin induced neuromast hair cell loss. ( PHENOTYPE:
|
|
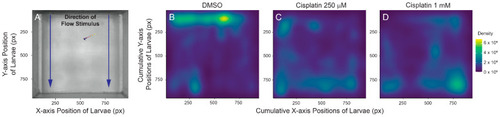
Cisplatin impairs larval ability to station hold in the presence of flow stimulus. ( PHENOTYPE:
|
|
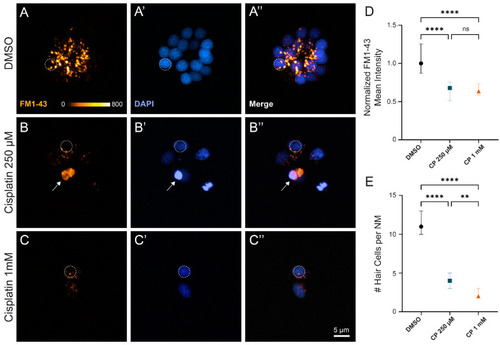
Surviving neuromast hair cells after cisplatin treatment demonstrate impaired mechanotransduction. ( |