- Title
-
CRISPR/Cas9-Mediated Constitutive Loss of VCP (Valosin-Containing Protein) Impairs Proteostasis and Leads to Defective Striated Muscle Structure and Function In Vivo
- Authors
- Voisard, P., Diofano, F., Glazier, A.A., Rottbauer, W., Just, S.
- Source
- Full text @ Int. J. Mol. Sci.
|
Generation of zebrafish vcp knockout by CRISPR/Cas9 gene editing. (A) Structure and partial sequence of the vcp gene and protein. CRISPR/Cas9 gene editing modifies exon 3 of vcp leading to the insertion of 206 nucleotides, a frame shift, and finally the introduction of a premature stop codon, and thereby the premature termination of Vcp translation after 138 amino acids. (B,C) Immunoblot analysis and quantification of homozygous mutant vcpex3/ex3 embryo protein lysate compared to protein lysate obtained from clutchmates (indicated as WT) at 72 hpf (N = 3; mean ± S.D, p ≤ 0.0001 determined using two-tailed t-test). Error bars indicate s.d.; **** p < 0.0001. (D) Quantitative real-time PCR of vcpex3/ex3 and WT embryos at 72 hpf shows no alterations in vcp transcript levels in vcpex3/ex3 embryos (N = 3, mean ± SD, p < 0.0001 determined using two-tailed t-test). Error bars indicate s.d.; ns, not significant. (E) In-crosses of heterozygous carriers yielded offspring demonstrating the regular Mendelian genotypic ratio of 25% homozygous wild-type (vcp+/+; WT), 50% heterozygous (vcp+/ex3), and 25% homozygous mutant (vcpex3/ex3) embryos (N = 3, n = 100). (F) A Kaplan–Meier survival curve was constructed for 11 homozygous vcpex3/ex3 mutants and 28 control embryos, which showed a sharp increase in mortality starting at 72 hpf in vcpex3/ex3 mutants compared to their wild-type clutchmates (n = 39, Mantel–Cox test, p < 0.0001). (G) Lateral view of brightfield images of WT and vcpex3/ex3 zebrafish embryos at 24, 48, and 72 hpf. Homozygous vcpex3/ex3 mutants exhibit phenotypic alterations at 72 hpf, whereas vcpex3/ex3 mutant embryos at 24 and 48 hpf are indistinguishable from their wild-type clutchmates. ns: not significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
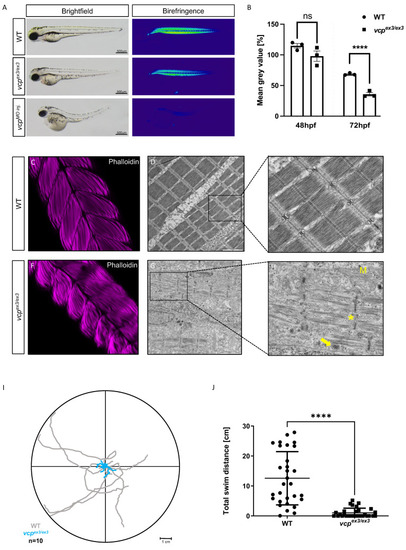
Genetic loss of vcp leads to skeletal muscle dysfunctions. (A) Lateral view of brightfield and birefringence images for WT, vcpex3/ex3, and VCP-MO-injected embryos at 72 hpf. (B) Densitometric analysis of the birefringence signal at 72 hpf reveals significantly reduced signal intensity in vcpex3/ex3 embryos, implying impaired skeletal muscle organization, whereas birefringence signal intensity is unaltered at 48 hpf (48 hpf: N = 3, n = 5/8, p = 0.6690; 72 hpf: N = 3, n = 8/15; p = 0.0001; two-tailed t-test). Error bars indicate s.d.; **** p < 0.0001; ns, not significant. (C,F) Confocal microscopic analysis of phalloidin-stained skeletal muscles of WT and vcpex3/ex3 embryos at 72 hpf. vcpex3/ex3 embryos show disrupted muscle fibers compared to the clutchmates. (D,E,G,H) Electron microscopic pictures of WT and vcpex3/ex3 skeletal muscle embryos at 72 hpf. vcpex3/ex3 embryos show damaged mitochondria (M) and increased electron-dense inclusion bodies (→) in addition to the disrupted muscle fibers (*). (I) Touch-evoked assay reveals reduction in responsiveness upon mechanical stimulus in vcpex3/ex3 embryos, as shown in the motion trace diagram. (J) Quantification of the total swim distance shows significantly reduced motility in vcpex3/ex3 compared to WT embryos (N = 3, n = 10, mean ± S.D; p < 0.0001; two-tailed t-test). Error bars indicate s.d.; **** p < 0.0001. ns: not significant. PHENOTYPE:
|
|
Genetic loss of vcp leads to heart failure in homozygous mutant embryos. (A,B) Analysis of the distribution of meromysin (MF20, green) and atrial-specific myosin heavy chain (S46, red) shows normal expression levels and patterns in vcpex3/ex3 embryos, suggesting regular heart chamber specification and cardiomyocyte differentiation. (C) Heart rate quantification at 48 hpf does not reveal any alterations in vcpex3/ex3 embryos (161 ± 15 heart beat/min) compared to age-matched clutchmates (165 ±10 heart beat/min) (N = 3, n = 11/12, mean ± S.D, p = 0.7000 determined using two-tailed t-test). At 72 hpf, vcpex3/ex3 embryos show highly significant reduction in the heart rate (N = 3, n = 9/12; vcpex3/ex3 0 heart beat/min; WT 150 ± 10 beats/min; mean ± S.D p ≤ 0.0001; two-tailed t-test). (D) Measurement of ventricular fractional shortening at 48 hpf shows no significant differences between WT (34.64 ± 8%) and vcpex3/ex3 (28.73 ± 7%) embryos (N = 3, n = 19/9, mean ± S.D; p = 0.1000; two-tailed t-test). At 72 hpf, fractional shortening is significantly reduced in vcpex3/ex3 embryos (N = 3, n = 12, mean ± S.D; FS vcpex3/ex3 3 ± 7%; FS WT 50.48 ± 10%; p ≤ 0.0002; two-tailed t-test). (E,F) Electron microscopic pictures of WT and vcpex3/ex3 hearts at 72 hpf. vcpex3/ex3 embryos show severely dysmorphic mitochondria (M) and disorganized myofibrils (*). ns: not significant. EXPRESSION / LABELING:
PHENOTYPE:
|
|
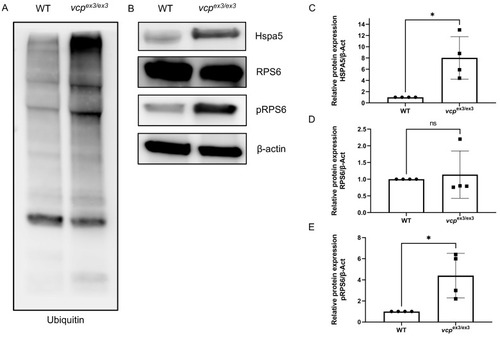
ER stress and impaired protein homeostasis in vcpex3/ex3 embryos. (A) Loss of Vcp leads to an accumulation of ubiquitinated proteins in vcpex3/ex3 embryos compared to WT controls (N = 4). (B,C) Significantly increased Hspa5 protein levels suggest ER stress in vcpex3/ex3 embryos (N = 4; p = 0.0286; two-tailed t-test). Error bars indicate s.d.; * p < 0.05. (B,D) Total RPS6 protein levels are unchanged in vcpex3/ex3 mutant embryos (N = 4; p = 0.0286; two-tailed t-test), whereas phosphoRPS6 protein levels. Error bars indicate s.d.; ns, not significant. (B,E) are significantly increased, suggesting an activation of mTORC1 signaling (N = 4; p = 0.0286; two-tailed t-test). ß-Actin was used as a loading control. Error bars indicate s.d.; * p < 0.05. ns: not significant. EXPRESSION / LABELING:
PHENOTYPE:
|