- Title
-
Novel Quorum Quenching YtnP Lactonase From Bacillus paralicheniformis Reduces Pseudomonas aeruginosa Virulence and Increases Antibiotic Efficacy in vivo
- Authors
- Djokic, L., Stankovic, N., Galic, I., Moric, I., Radakovic, N., Šegan, S., Pavic, A., Senerovic, L.
- Source
- Full text @ Front Microbiol
|
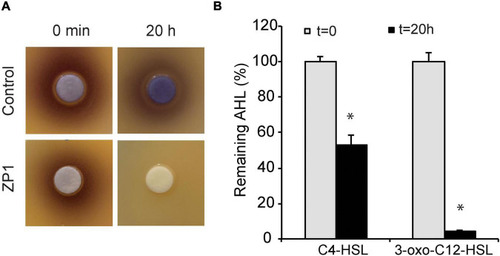
Determination of lactonase activity. (A) Hydrolysis of C6-HSL. (B) Hydrolysis of C4-HSL and 3-oxo-C12-HSL. Values are plotted relative to buffer-treated control and are representative of two independent experiments ± SD. *P < 0.05. |
|
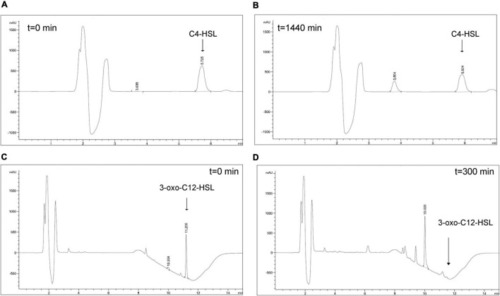
HPLC analysis of AHLs degradation by YtnP-ZP1. Main ion peaks of AHLs standards (t = 0) (A,C), as well as ring-opened products of AHLs (B,D) are presented. |
|
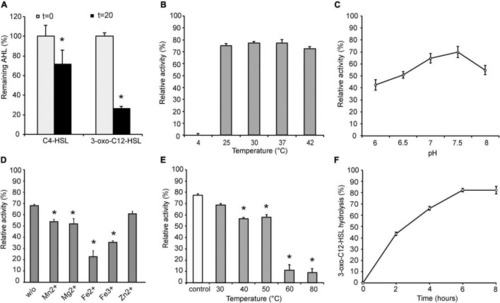
Characterization of YtnP-ZP1. Enzyme specificity (A) and the effects of temperature (B), pH (C), metal ions (D), pre-incubation temperature (E) on lactonase activity and the rate of 3-oxo-C12-HSL hydrolysis (F). w/o, control reaction without metal ions; control, reaction with enzyme without pre-incubation. Values are representative of three independent experiments ± SD. *P < 0.05. |
|
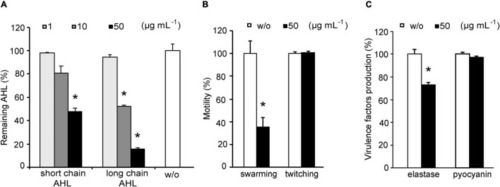
Effects of YtnP-ZP1 on P. aeruginosa PAO1 lactones (A), motility (B), and on elastase and pyocyanin production (C). Values are plotted relative to buffer-treated control (w/o) and are representative of three independent experiments ± SD. *P < 0.05. |
|
Effects of YtnP-ZP1 on P. aeruginosa PAO1 biofilms. Biofilm formation in the absence (w/o) or presence of lactonase (A). Inhibition of P. aeruginosa mature biofilms. Values are plotted relative to buffer-treated control (w/o) (B). Values are representative of three independent experiments ± SD. *P < 0.05. |
|
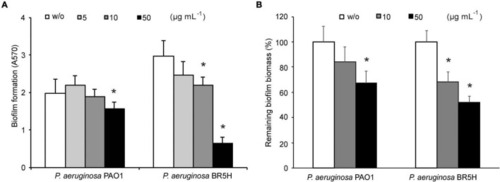
Effects of YtnP-ZP1 on biofilm formation in environmental isolates. Values are plotted relative to buffer-treated control (w/o) and are representative of three independent experiments ± SD. *P < 0.05. |
|
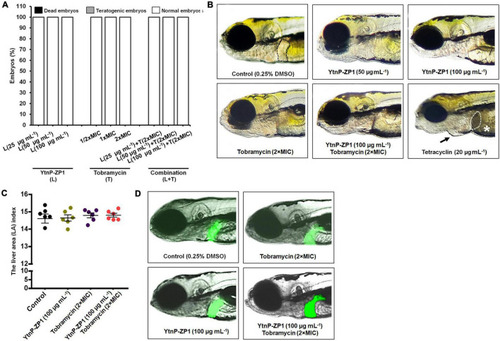
In vivo toxicity evaluation of YtnP-ZP1, tobramycin, and their combination in the zebrafish (Danio rerio) model. Acute toxicity was assessed using wild-type embryos exposed to the different doses of enzyme, antibiotic, and their combination (A). Embryos were treated at 6 hpf and evaluated for survival, teratogenicity, cardiotoxicity, and hepatotoxicity at 120 hpf (n = 60 per a dose). Normally developed embryos, without signs of hepato- and cardiotoxicity, after exposure to 100 μg mL–1 of YtnP-ZP1, 2 × MIC of tobramycin and the combination of 100 μg mL–1 lactonase and 2 × MIC tobramycin are shown (B). The normal (clear) liver in a control embryo is outlined. Tetracycline caused liver necrosis and darkening (dashed outlined), yolk necrosis (asterisk), and pericardial edema (arrow). The hepatotoxicity of the tested enzyme and its combination with antibiotic was additionally verified in zebrafish embryos with fluorescently labeled liver (n = 6) according to the liver fluorescence (C) and the liver area index (D). |
|
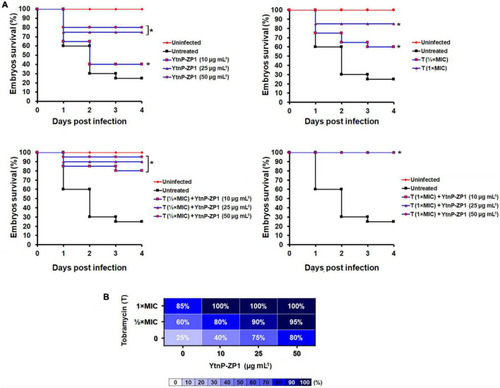
YtnP-ZP1 efficiently rescued the zebrafish embryos of the lethal P. aeruginosa PAO1-GFP-infection. Wild-type zebrafish embryos were infected with P. aeruginosa PAO1-GFP in the circulation valley and incubated at 32°C (n = 20 per dose). The Kaplan–Meier survival curves (A) and the survival heat map at 120 hpf (B) of P. aeruginosa PAO1-infected embryos upon the different treatments are shown. *P < 0.05. |
|
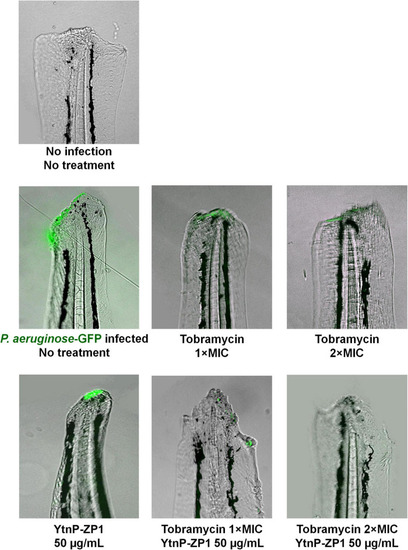
Pseudomonas aeruginosa PAO1 tail wound infection was successfully eliminated by applying YtnP-ZP1 in combination with tobramycin. Wild-type zebrafish embryos were decapitated at 72 hpf and infected with 4–6 × 107 cells of P. aeruginosa PAO1-GFP. After 1–2 h of recovery, wounded embryos were treated with tobramycin, lactonase, or their combination. Individual treatment with enzyme or antibiotic partially eliminated infection from the wounded tail, while combination treatment with lactonase and tobramycin was more effective resulting in no visible bacterial infection (lack of fluorescence). |