- Title
-
Brief Developmental Exposure to Fluoxetine Causes Life-Long Alteration of the Brain Transcriptome in Zebrafish
- Authors
- Nozari, A., Gagné, R., Lu, C., Yauk, C., Trudeau, V.L.
- Source
- Full text @ Front Endocrinol (Lausanne)
|
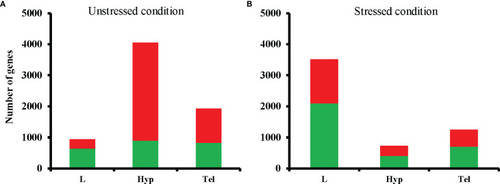
Total numbers of differentially expressed genes (DEGs) in fluoxetine-exposed groups compared to respective controls in unstressed |
|
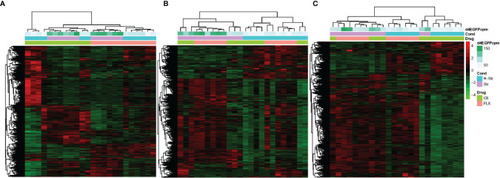
The effect of developmental fluoxetine exposure on transcriptome patterns in larval and adult male zebrafish central nervous system in the unstressed and stressed conditions. Hierarchical clustering (Spearman correlation) is shown for all 24 samples (stressed, unstressed, fluoxetine-exposed, and control ethanol-exposed) in a single pooled tissue sample (i.e., larval head, hypothalamus, and telencephalon) and their relation to FLX and stress. |
|
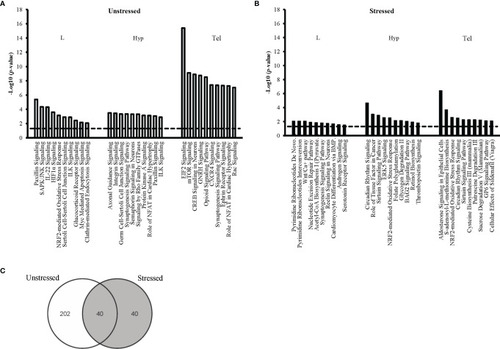
Top ten affected canonical pathways following early-life exposure to fluoxetine. The ten pathways with the highest level of significance (i.e. lowest p-value) for each target tissue are shown in larval heads (L), hypothalmus (Hyp), and telencephalon (Tel) in the unstressed |
|
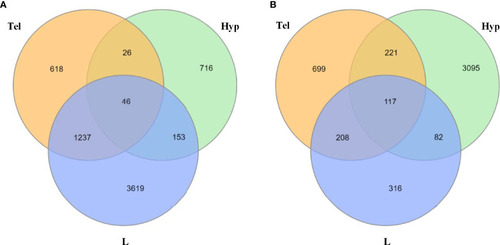
Number of common dysregulated genes in larvae and adult zebrafish upon developmental exposure to fluoxetine in stressed and unstressed conditions. |