|
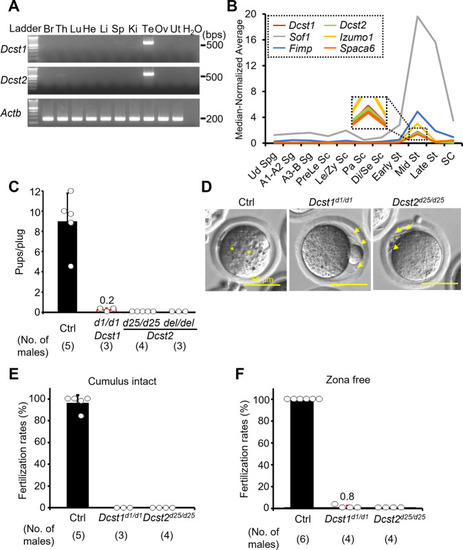
Male fertility of <italic>Dcst1</italic> and <italic>Dcst2</italic> mutant mice.A Multi-tissue gene expression analysis. Dcst1 and Dcst2 are abundantly expressed in the mouse testis. Beta actin (Actb) was used as the loading control. Br, brain; Th, thymus; Lu, lung; He, heart; Li, liver; Sp, spleen; Ki, kidney; Te, testis; Ov, ovary; Ut, uterus. B Median-normalized level of Dcst1 and Dcst2 mRNA expression during mouse spermatogenesis. Dcst1 and Dcst2 are strongly expressed in mid-round spermatids, corresponding to other fusion-related factors. Ud Sg, undifferentiated spermatogonia; A1-A2 Sg, A1-A2 differentiating spermatogonia; A3-B Sg, A3-A4-In-B differentiating spermatogonia; Prele Sc, preleptotene spermatocytes; Le/Zy Sc, leptotene/zygotene spermatocytes; Pa Sc, pachytene spermatocytes; Di/Se Sc, diplotene/secondary spermatocytes; Early St, early round spermatids; Mid St, mid round spermatids; Late St, late round spermatids; SC, Sertoli cells. C Male fecundity. Each male was caged with 2 wild-type females for >1 month. Dcst2d25/wt and del/wt males were used as the control (Ctrl). Dcst1d1/d1, Dcst2d25/d25, and Dcst2del/del males succeeded in mating [number of plugs: 19 (Ctrl), 17 (Dcst1d1/d1), 42 (Dcst2d25/d25), 24 (Dcst2del/del)], but the females very rarely delivered pups [pups/plug: 9.0 ± 2.8 (Ctrl), 0.2 ± 0.2 (Dcst1d1/d1), 0 (Dcst2d25/d25), 0 (Dcst2del/del)]. D Egg observation after IVF. After 8 h of incubation, pronuclei were observed in the control heterozygous sperm (asterisks). However, Dcst1 KO and Dcst2 KO sperm accumulated in the perivitelline space (arrows). The scale bars are 50 μm. E Sperm fertilizing ability using cumulus-intact eggs in vitro. Dcst1 KO and Dcst2 KO sperm could not fertilize eggs [fertilization rates: 96.5 ± 7.1% (Ctrl, 231 eggs), 0% (Dcst1d1/d1, 97 eggs), 0% (Dcst2d25/d25, 197 eggs)]. F Sperm fertilizing ability using ZP-free eggs in vitro. Dcst1 KO and Dcst2 KO sperm rarely fertilized eggs [fertilization rates: 100% (Ctrl, 142 eggs), 0.8 ± 1.6% (Dcst1d1/d1, 94 eggs), 0% (Dcst2d25/d25, 88 eggs)]. All values in this figure are shown as the mean ± SD.
|