- Title
-
High-Resolution, 3D Imaging of the Zebrafish Gill-Associated Lymphoid Tissue (GIALT) Reveals a Novel Lymphoid Structure, the Amphibranchial Lymphoid Tissue
- Authors
- Dalum, A.S., Kraus, A., Khan, S., Davydova, E., Rigaudeau, D., Bjørgen, H., López-Porras, A., Griffiths, G., Wiegertjes, G.F., Koppang, E.O., Salinas, I., Boudinot, P., Rességuier, J.
- Source
- Full text @ Front Immunol
|
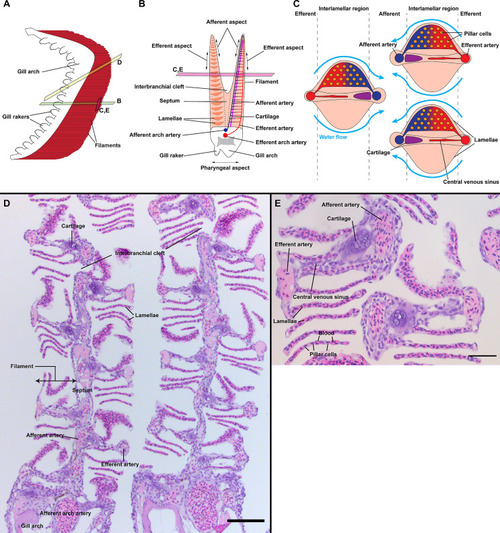
General organization of zebrafish gills. Schematic representations of the overall anatomy of adult zebrafish gills as observed from the side |
|
General organization of the zebrafish GIALT. Representative deconvolved confocal images of adult zebrafish gills acquired from a transversal |
|
The four zebrafish gill arches display the same lymphoid organization. Representative deconvolved confocal images of an adult zebrafish branchial cavity showing the 4 gills arches with transversal orientation |
|
Organization of the zf-GIALT above the gill arch. (Sub-regions 1-3). Representative deconvolved confocal high-resolution images of adult zebrafish gills acquired with a transversal |
|
Organization of the zf-GIALT at the gill arch. (Sub-regions 4-5). Representative deconvolved confocal high-resolution images of adult zebrafish gills acquired with a transversal orientation. Images were acquired from 30 μm whole-body cryosections stained with phalloidin (green) and DAPI (blue) and where T/NK cells were labeled with anti-ZAP70 antibody (red hot). The localization of the GIALT sub-regions is illustrated in |
|
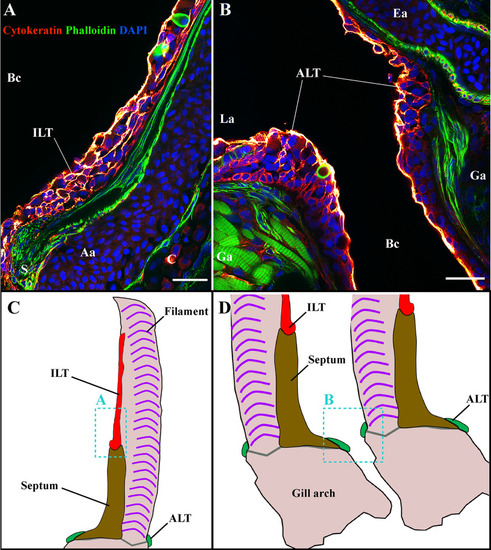
The amphibranchial lymphoid tissue is a continuous structure along gill arches. Representative deconvolved confocal images of an adult zebrafish gill arch observed from the side. The images were acquired from wholemount dissected gill arches stained with phalloidin (green) and DAPI (blue) and where T/NK cells were labeled with anti-ZAP70 antibody (red hot). |
|
The ILT and ALT are structured by a complex network of reticulated epithelial cells. Representative deconvolved confocal images of adult zebrafish gills displaying the ILT |
|
General organization of the structure lymphoid tissues in zebrafish gills. Schematic representations of the localization of the ALT (green) and ILT (yellow) within adult zebrafish gills. The ALT runs along the base of filaments while the ILT is found on the inner (afferent) aspect of filaments, on top of the interbranchial septum. |
|
Antigen-presenting cells and granulocytes are also present within the ILT and ALT. Representative deconvolved confocal images of adult zebrafish gills displaying the ILT |
|
T/NK cell proliferation and RAG2 expression. Representative deconvolved confocal images of adult zebrafish gills displaying the ILT |
|
Modifications of the zf-GIALT structure upon SVCV infection. Representative deconvolved confocal images of adult zebrafish gills 3 days |
|
The ALT is a preserved structure between distant teleost species. Representative acquisitions of anti-ZAP70 (brown/red hot) labeled gills section, from different fish species, displaying the side of a gill arch, at the base of filaments. |