- Title
-
KYNA/Ahr Signaling Suppresses Neural Stem Cell Plasticity and Neurogenesis in Adult Zebrafish Model of Alzheimer's Disease
- Authors
- Siddiqui, T., Bhattarai, P., Popova, S., Cosacak, M.I., Sariya, S., Zhang, Y., Mayeux, R., Tosto, G., Kizil, C.
- Source
- Full text @ Cells
|
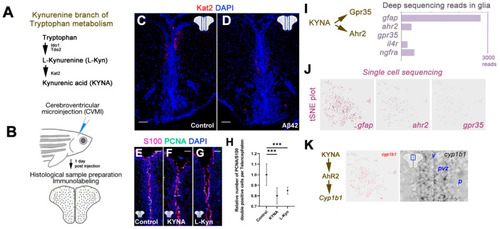
Kynurenic acid signaling is through Ahr2 in adult zebrafish brain. (A) Catabolic cascade from tryptophan to kynurenic acid. (B) Cerebroventricular microinjection paradigm. (C,D) Immunohistochemical staining for Kat2 in control (C) and Aβ42-injected (D) adult zebrafish brain sections. (E–G) Immunohistochemical staining for S100 and PCNA in control (E), KYNA-injected (F) and L-Kynurenine-injected (G) zebrafish brain sections. (H) Quantification of proliferating glial cells of conditions in (E–G). (I) Deep sequencing reads of KYNA receptors ahr2 and gpr35 as well as other receptors of neural stem cells, il4r and ngfra. Reads for gfap is for comparison. (J) tSNE plots from single cell sequencing in adult zebrafish telencephalon in progenitor cells. Expression of gfap, ahr2 and gpr35 shown. ahr2 is the only receptor for KYNA in progenitor cells. (K) Ahr2 signaling regulates cyp1b1 expression. Cyp1b1 is present in progenitor cells as determined in single cell sequencing, and in situ hybridization. Scale bars equal 50 μm. Statistical calculations and values are provided in Table S1. |
|
Ahr2-dependent cyp1b1 expression is regulated by Aβ42 and KYNA in the adult zebrafish brain. (A) Normalized expression levels of cyp1b1 in zebrafish telencephalon injected with control vehicle (PBS), KYNA, BNF, Ahr antagonist or Aβ42. (B) In situ hybridization for cyp1b1 in control, BNF, KYNA, antagonist and Aβ42 injected brains. Note the reduction of expression with the Ahr antagonist and Aβ42 and increase with BNF or KYNA. Scale bars equal 50 μm. Statistical calculations and values are provided in Table S1. |
|
KYNA/Ahr2 signaling regulates neural stem cell proliferation in adult zebrafish brain. (A) Immunohistochemical stains for S100β (red) and PCNA (green) in control, BNF, KYNA, Aβ42 and Ahr antagonist injected brains. (B) Quantification of relative numbers of proliferating glial cells in conditions in A. Scale bars equal 50 μm. Statistical calculations and values are provided in Table S1. PHENOTYPE:
|
|
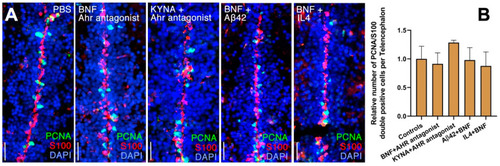
Ahr2-dependent suppression of neural stem cell plasticity is antagonized by neuroregenerative factors in adult zebrafish brain. (A) Immunohistochemical stains for S100β (red) and PCNA (green) in control (PBS), BNF+Ahr antagonist, KYNA+Ahr antagonist, BNF+Aβ42 and BNF+IL4 injected brains. (B) Quantification of relative numbers of proliferating glial cells in conditions in A. Scale bars equal 50 μm. Statistical calculations and values are provided in Table S1. PHENOTYPE:
|
|
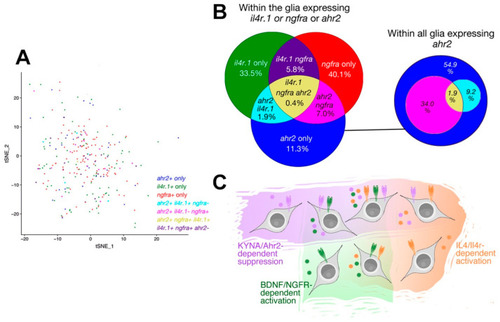
Single cell sequencing-based co-expression of il4r, ngfra, and ahr2 in adult zebrafish brain. (A) tSNE plot for astroglial cells in adult zebrafish telencephalon showing the cells expressing ahr2, il4r, ngfra and all possible triple combinations. (B) Venn diagram for the abundance of progenitor cells expressing different combinations. (C) Schematic depiction of various subtypes of neural stem cells that are responsive to multiple signaling pathways. KYNA/Ahr2-dependent regulation marks a new functional subtype of neural stem cells in zebrafish brain. |
|
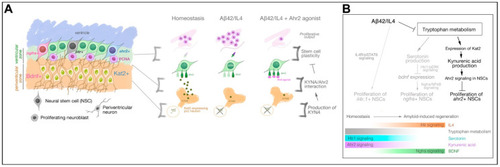
KYNA/Ahr2 signaling defines the third distinct Alzheimer’s disease-responsive subset of neural stem cell population in adult zebrafish brain (A) Schematic view of the regulation of neural stem cell plasticity and neurogenesis in adult zebrafish brain by KYNA/Ahr2 signaling. (B) Consolidated view of the effects of Aβ42 on neural stem cell plasticity. Aβ42 reduces the expression levels of KAT2, which generates KYNA, an inhibitory regulator of neural stem cell proliferation through its receptor Ahr2. For successful regeneration to take place, tryptophan metabolism is suppressed and IL4/BDNF-mediated signaling mechanisms are enhanced. |