- Title
-
Anterior expansion and posterior addition to the notochord mechanically coordinate embryo axis elongation
- Authors
- McLaren, S.B.P., Steventon, B.J.
- Source
- Full text @ Development
|
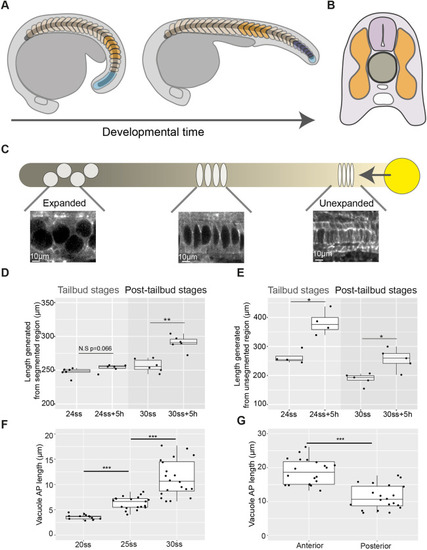
Axis elongation occurs predominantly in the unsegmented region during tailbud stages and later continues in segmented tissue in zebrafish embryos. (A) Schematic outlining segmented (orange and dark-blue) and unsegmented (light-blue) regions of the somitic compartment. Dark-blue somites are formed from unsegmented tissue as development progresses. (B) Schematic showing axial tissues in a cross-section of the zebrafish embryo. The notochord (dark-grey) is flanked on either side by the somitic compartment (orange) and dorsally by the neural tube (light-purple). (C) Schematic outlining events in notochord morphogenesis. Vacuolation progresses from the anterior (darker) to posterior (lighter) of the notochord and progenitors expressing noto (yellow) add cells to the notochord rod. (D) Length of a region of segmented tissue in tailbud (24ss) and post-tailbud (30ss) embryos and length generated from this region over 5 h (n=6 and n=6, respectively; **P<0.01, Mann–Whitney U test). (E) Length of a region of unsegmented tissue in tailbud (24ss) and post-tailbud (30ss) embryos and length generated from this region over 5 h (n=4 and n=5 embryos per stage, respectively; *P<0.05, Mann–Whitney U test). (F) AP length of vacuoles at different stages of development (n=3, n=4 and n=4 embryos per stage, respectively, 3-5 vacuoles measured per embryo; ***P<0.001, Kruskal–Wallis test). (G) AP length of vacuoles in anterior and posterior regions of 30ss embryos (n=4 embryos, 5 vacuoles measured per region; ***P<0.001, Mann–Whitney U test). Anterior is to the left and posterior to the right in all images. N.S., not significant. |
|
|
|
|
|
|