- Title
-
Crocetin and Its Glycoside Crocin, Two Bioactive Constituents From Crocus sativus L. (Saffron), Differentially Inhibit Angiogenesis by Inhibiting Endothelial Cytoskeleton Organization and Cell Migration Through VEGFR2/SRC/FAK and VEGFR2/MEK/ERK Signaling Pathways
- Authors
- Zhao, C., Kam, H.T., Chen, Y., Gong, G., Hoi, M.P., Skalicka-Woźniak, K., Dias, A.C.P., Lee, S.M.
- Source
- Full text @ Front Pharmacol
|
Chemical structures of (A) crocetin and (B) crocin. |
|
Crocetin and crocin inhibited SIV formation in |
|
Representative HPLC profiles for |
|
Cytotoxicity of crocetin and crocin on HUVECs (A) HUVECs were treated with different concentrations of crocetin (0.2, 0.3, 0.5, and 1 mM) and |
|
Crocetin and crocin inhibited endothelial cell migration and capillary-like tube formation. |
|
Crocetin and crocin inhibited the activation of VEGFR2 and its downstream signaling pathways. HUVECs were starved for 2 h and then pretreated with crocetin (10, 20 and 40 μM) or crocin (100, 200 and 400 μM) for 4 h before being stimulated by VEGF (50 ng/ml) for 15 min. Western blot assay was used for investigating the expression levels of the major proteins involved in VEGF-mediated angiogenesis signaling in HUVECs. Crocetin and crocin down-regulated the expression levels of |
|
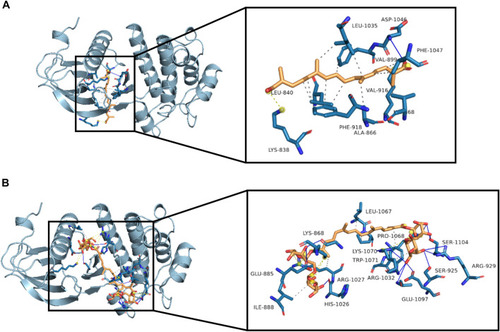
Molecular interactions between crocetin or crocin with VEGFR2. Three-dimensional view of crocetin and crocin located in the binding site of VEGFR2 are shown in |
|
Crocetin and crocin inhibited angiogenesis |