- Title
-
Pyramidal Neurons of the Zebrafish Tectum Receive Highly Convergent Input From Torus Longitudinalis
- Authors
- DeMarco, E., Tesmer, A.L., Hech, B., Kawakami, K., Robles, E.
- Source
- Full text @ Front. Neuroanat.
|
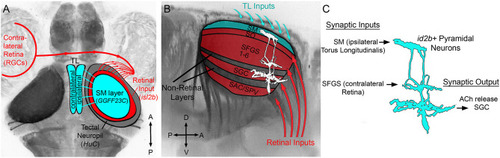
Overview of TL-tectum circuit and PyrN connectivity. |
|
|
|
Axonal projection from TL to tectum is glutamatergic. (A) Dorsal view confocal image of EGFP and DsRed fluorescence in the brain of a Tg(hspGGFF23C,uas:egfp,vglut2b:rfp) larva. (B) Magnified views of region indicated by box in (A) showing the EGFP (B), DsRed (B′), and merged (B′′) fluorescence channels. Arrows indicate neurons with EGFP and DsRed colocalization. (C) Van Steensel's cross-correlation functions of EGFP and DsRed overlays. Local maxima of Pearson's Coefficient (PC) near ΔX = 0 indicates colocalization. N = 10 larvae. (D) Dorsal view confocal image of the tectum in a 7 dpf Tg(hspGGFF23C,uas:egfp) larva fixed and immunofluorescently stained with anti-EGFP antibody. Note dense plexus of EGFP-positive axons in SM. (E) Side-view of a single tectal lobe from tectum in (D), including immunofluorescent staining with anti-VGluT1 antibody in red channel. Note superficial EGFP labeling in SM layer and punctate VGluT1 immunofluorescence. Note increased density of puncta in SM layer. (F) High-resolution dorsal view of a SM subregion within tectum in (D). Note overlap between VGluT1 puncta and EGFP-positive plexus and lack of VGluT1 labeling in EGFP-negative regions. (G) Van Steensel's cross-correlation functions of EGFP and DsRed overlays for image in (F′). Local maxima of Pearson's Coefficient (PC) near ΔX = 0 indicates colocalization. N = 4 larvae. Scale bar: 150 μm in (A), 30 μm in (B,E), 75 μm in (D), and 15 μm in (F). |
|
PSD95-positive post-synaptic specializations in PyrN dendrites. (A) Maximum projection of PSD95-EGFP and DsRed expression in an isolated id2b:gal4 positive PyrN. Note discrete enrichments of PSD95-EGFP relative to cytosolic DsRed label. (A′) +50° Y-axis rotated view of image volume in (A). SM, SFGS, and SGC dendritic portions are indicated by boxed regions. Note presence of PSD95-EGFP puncta in SM, SFGS, and SGC layer dendrites. (B) −40° Y-axis rotated views of isolated subvolumes from boxed regions in (A′) showing PSD95-EGFP and DsRed expression in the SM, SFGS, and SGC dendrites. (C) Threshold images of PSD95-EGFP channel in rotated subvolumes in (B) as an example of threshold values used for automated particle analysis (puncta counting). (D–F) Quantification of PSD95-EGFP puncta number, neurite length, and puncta density for SM, SFGS, and SGC dendrite subregions in 7 fully reconstructed PyrNs obtained from 7 larvae. Scale bar: 20 μm in (A) and 15 μm in (B,C). *p < 0.05; **p < 0.001; ANOVA with Tukey's post-hoc test. |
|
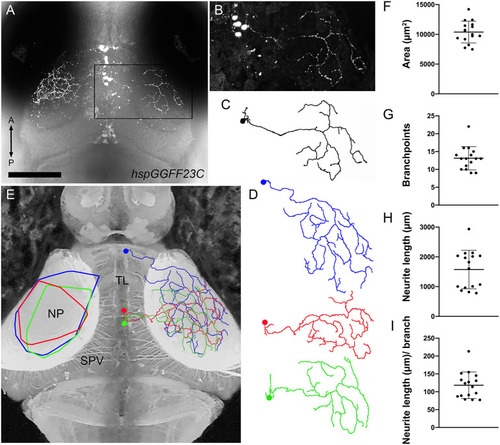
Morphology of individual TL axons innervating SM. |
|
Morphometry of PyrN arbors formed in SM, SFGS, and SGC layers of tectum. |
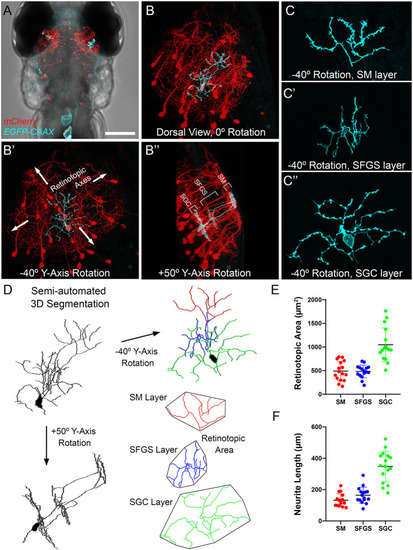
|
Evidence for high degree of convergence from TL inputs to PyrNs. (A) Maximum projection image of fluorescence in a 7 dpf Tg(HuC:lynTagRFP-t) larva. (B) Maximum projection of boxed subregion in (A) rotated −40° about the Y-axis, an orientation orthogonal to the tectal layers used for retinotopic area measurements. Maximum projection of equivalent subregions in a 7 dpf Tg(hspGGFF23C,uas:egfp,isl2b:TagRFP) larva rotated −40° about the Y-axis. Yellow polygon drawn to assist in comparison of area differences between the three images. (C) Schematic at left summarizes tectal inputs from TL-recipient SM layer (cyan), retinorecipient layers (red), and total tectal neuropil area. Schematic at right summarizes area measurements for these different neuropil layers. (D) Skeletonized tracings and average retinotopic areas for TL axons, RGC axons, and PyrN neurite subregions. (E) Implications of size disparity between TL axon arbors and retinal axon arbors in tectum. TL axon arbor areas are depicted as cyan circles and RGC axon arbor areas are red ovals. Due to the large size of TL arbors, a PyrN dendrite at the center of SM has the potential to contact all TL inputs. Conversely, the small relative size of RGC axons innervating SFGS means that a PyrN dendrite at the center of SFGS has the potential to contact only a small fraction of retinal inputs to SFGS. Due to the small size of both pre- and post-synaptic elements this remains true even as the number of RGC inputs increases. Scale bar: 200 μm in (A) and 100 μm in (B). |