- Title
-
Graphene oxide nanosheets modulate spinal glutamatergic transmission and modify locomotor behaviour in an in vivo zebrafish model
- Authors
- Cellot, G., Vranic, S., Shin, Y., Worsley, R., Rodrigues, A.F., Bussy, C., Casiraghi, C., Kostarelos, K., McDearmid, J.R.
- Source
- Full text @ Nanoscale Horiz
|
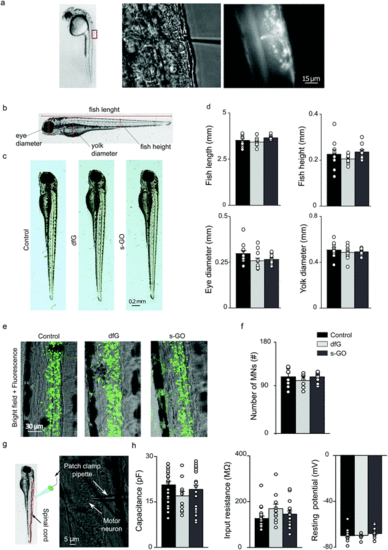
Intra-spinal delivery of s-GO and dfG does not alter zebrafish development, neuronal survival or the electrophysiological basic properties of motor neurons. (a) Experimental setting for injections of nanomaterials in the spinal cord of 2 dpf zebrafish. Left: Lateral view of a 2 dpf zebrafish larva; the rectangle indicates the region of the body targeted by injection. Middle: Bright field image at higher magnification of the dorsal part of fish where solutions are delivered through a tiny glass pipette. Right: Fluorescence field of the previous image, showing that the injected solutions, added with a fluorescent dye, are delivered precisely in the spinal cord. (b) Schematic representation of the anatomical traits measured in zebrafish to analyse their development. (c) Bright field images of control, dfG-injected and s-GO-injected fish two days after injection. (d) Plots reporting the values of fish length and height, eye and yolk diameters. (e) Confocal images (Z-stack reconstructions) of the spinal cord of control, dfG-injected and s-GO-injected fish. Green signal is GFP expressed in motor neurons. (f) Plots reporting the number of motor neurons in the two segments of spinal cord around the site of injection. (g) Experimental setting for in vivo patch clamp recordings from motor neurons in zebrafish spinal cord: on the left a schematic representation of the patch clamp pipette inserted in the spinal cord (in red) of a larva, on the right bright field image of spinal neurons during recordings. A patch clamp pipette targeting the cell body of a motor neuron is visible. (h) Plots reporting the capacitance, the input resistance and the resting membrane potential of primary motor neurons. Dots superimposed to bars correspond to single fish or single neuron values. |
|
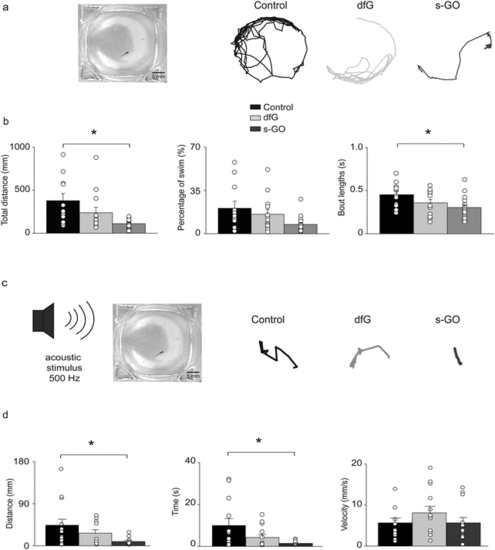
Effects of intra-spinal delivery of s-GO on zebrafish behaviour. (a) Left: Bright field image of recording chamber for free swimming test. Right: Swimming trajectories of 4 dpf control, dfG-injected and s-GO-injected zebrafish recorded over a 5 min period. (b) Plots reporting the values of the total distance swum, percentage of time spent swimming and swimming bout length. Dots superimposed to bars correspond to single experiments values. s-GO treatment significantly decreased the total distance swum and the length of swimming bouts (*P < 0.05). (c) Left, sketch of the experimental setting in startle response experiments. Right, swimming trajectories of 4 dpf control, dfG injected and s-GO-injected zebrafish swum after the application of an acoustic stimulus. (d) Plots reporting the values of the distance swum, the time spent swimming and the swimming velocity. s-GO treatment significantly decreased the distance swum and the time spent swimming (*P < 0.05). |
|
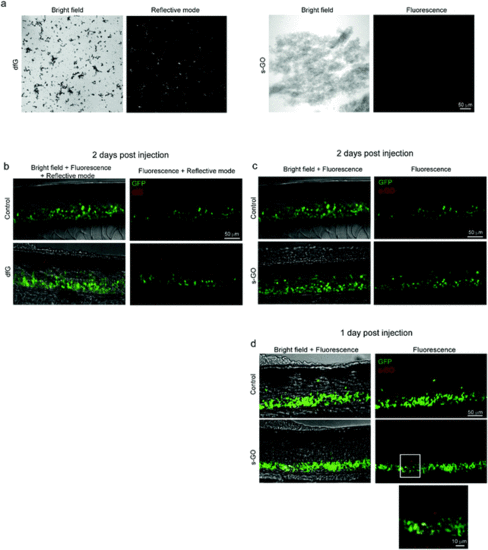
Confocal microscopy of dfG and s-GO injected in the spinal cord of zebrafish. (a) Images of dfG and s-GO dispersed in saline solution. Left panels for each material are bright field images, while right panels are images in reflective mode for dfG and in fluorescence for s-GO. (b) Z-stack reconstructions of the spinal cord of control and dfG-injected fish, 2 days after treatment. Images, acquired in fluorescence and reflective modes sequentially, show the localization of dfG (red) in the spinal cord, which is identifiable thanks to the presence of GFP positive motor neurons (green). (c and d) Z-stack reconstructions of the spinal cord of control and s-GO-injected fish, 2 days (c) and 1 day (d) after treatment. Images, acquired in fluorescence mode sequentially for the red and green channels, show the localization of s-GO (red) in the spinal cord, which is identifiable thanks to the presence of GFP positive motor neurons (green), only 1 day after the injection (d), but not later (c). In (d), below, magnification of the area in the square for s-GO injected fish. |