- Title
-
Endothelial Autophagy: an Effective Target for Radiation-induced Cerebral Capillary Damage
- Authors
- Ai, X., Ye, Z., Yao, Y., Xiao, J., You, C., Xu, J., Huang, X., Zhong, J., Fan, M., Song, X., Shi, H., Zhang, D., Zhao, C.
- Source
- Full text @ Sci. Rep.
|
Whole brain radiations decrease the density of cerebral vessels in human patient. ( |
|
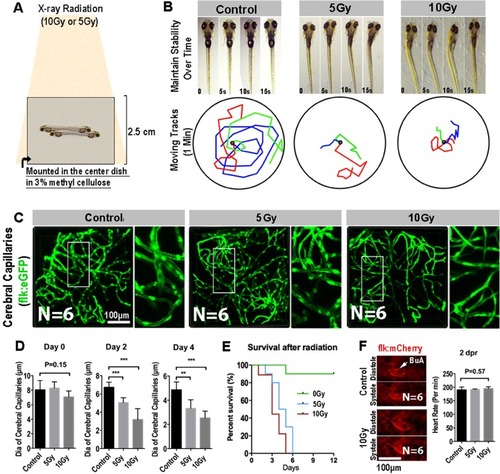
Radiation specifically damages the brain capillaries of transgenic zebrafish. ( |
|
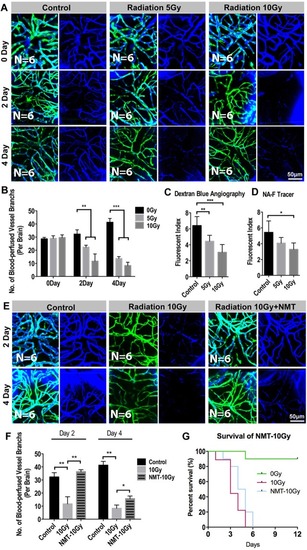
Radiation-induced endothelial damage results insufficient blood perfusion into the brain. ( |
|
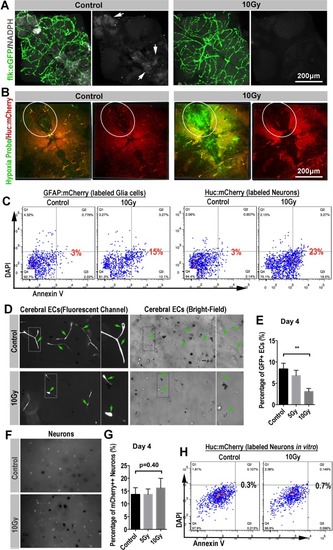
Secondary blood-perfusion insufficiency induced the apoptosis of neuron and glial ( |
|
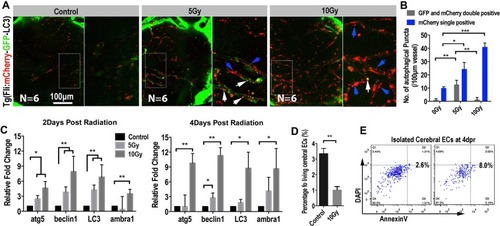
Extensive endothelial autophagy was induced by the radiation in the brain. ( |
|
Inhibition of autophagy enhanced the blood perfusion into the brain b ( |