- Title
-
The Alternative Splicing Regulator Nova2 Constrains Vascular Erk Signaling to Limit Specification of the Lymphatic Lineage
- Authors
- Baek, S., Oh, T.G., Secker, G., Sutton, D.L., Okuda, K.S., Paterson, S., Bower, N.I., Toubia, J., Koltowska, K., Capon, S.J., Baillie, G.J., Simons, C., Muscat, G.E.O., Lagendijk, A.K., Smith, K.A., Harvey, N.L., Hogan, B.M.
- Source
- Full text @ Dev. Cell
|
nova2uq14bh Mutants Exhibit Increased Lymphatic Sprouting (A and B) Overall morphology of a sibling (A) and nova2uq14bh mutant (B) at 54 hpf. (C–F) Trunk vasculature labeled with Tg(fli1a:nEGFP)y7;Tg(5.2lyve1b:DsRed)nz101 in a sibling (C) and (E) and a nova2uq14bh mutant (D) and (F) at 54 hpf (venous and LECs in red). Arrowheads indicate LEC nuclei. Venous intersegmental vessel (vISV) and PL. (G and H) Tg(fli1ep:lifeact-EGFP) labeling endothelial F-actin in PLs of a sibling (G) and a nova2uq14bh mutant (H) at 54 hpf. Arrowheads show exploratory PLs. (I) EC numbers within different lineages: dorsal aorta (DA, arterial), posterior cardinal vein (PCV, venous), and lymphatic ECs (LECs) at 54 hpf. Quantification across 5 body segments (siblings n = 17, mutants n = 17). (J) Quantification of nuclear ellipticity of ECs in the DA and PCV at 54 hpf. Quantification across 5 body segments (siblings n = 11, mutants n = 11). (K) Quantification of hyperactive PLs (defined as displaying cellular extensions that probe dorsal to the horizontal myoseptum [HM]) in siblings and nova2uq14bh mutants across 3 body segments (siblings n = 10, mutants n = 10). Light gray indicates wild-type morphology, dark gray indicates exploratory PLs in 1/2 body segments scored, and black indicates exploratory PLs in 2/2 body segments scored (p = 0.0174). Error bars represent SD, t test (I), or Mann-Whitney-test (J) and (K), ∗∗p < 0.01, ∗∗∗∗p < 0.0001, and ns; not significant; Scale bar represents 1 mm in (A) and (B), 25 μm in (C)–(F), and 20 μm in (G) and (H). See also Figures S1 and S2.
|
|
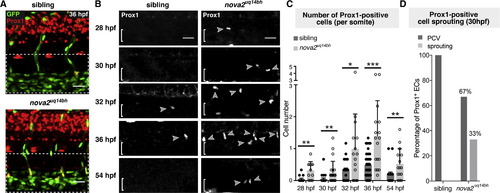
Increased, Premature, and Prolonged LEC Specification in Nova2 Mutants (A and B) Immunofluorescence (IF) staining of Prox1 at 28, 30, 32, 36, and 54 hpf in Tg(fli1a:nEGFP)y7 siblings and nova2uq14bh mutants. Arrowheads indicate Prox1-positive ECs (brackets indicate PCV). Left hand images show merged zoom out of trunk as an overlay of Prox1 and fli1a:nEGFP, which was used to confirm nuclear localization. (C) Quantification of the number of Prox1-positive ECs at 28 hpf (siblings n = 14 and mutants n = 11), 30 hpf (siblings n = 23 and mutants n = 22), 32 hpf (siblings n = 18 and mutants n = 15), 36 hpf (siblings n = 40 and mutants n = 17), and 54 hpf (siblings n = 15 and mutants n = 15). For all stages, 7 body segments were quantified except at 54 hpf when 6 body segments were used (see also Figures S3A–S3E). (D) Quantification of the number of Prox1-positive ECs in the PCV wall or having sprouted dorsally in 30 hpf sibling and mutant embryos. n = 7 Prox1-positive ECs in 4/23 embryos for siblings, and n = 33 Prox1-positive ECs in 14/22 embryos for mutants, with 11/33 Prox1-positive ECs sprouting from the PCV. Error bars represent SD, Mann-Whitney-test (C), ∗p < 0.05 ∗∗p < 0.01, and ∗∗∗p < 0.001; Scale bar represents 25 μm in (A) and (B). See also Figure S3. |
|
Nova2 Acts Cell Autonomously in Endothelial Cells (A) Expression of nova2 in the zebrafish trunk at 32 hpf by ISH. NT, neural tube. (B) Overview of Tg(nova2:YFP)uq40bh BAC transgenic line at 48 hpf. Inset = region of trunk vasculature displaying positive neurons, DA and PCV. (C) Tg(nova2:YFPuq40bh; fli1a:H2BmCherryuq37bh) double transgenic line at 36 hpf (C) and 60 hpf (Cʹ). (D) Nuclear Tg(nova2:YFP)uq40bh signal. H2BmCherry signal was used as a mask to limit analysis to the nucleus. Representative of n = 10 embryos analyzed at 36 hpf (D) and 60 hpf (Dʹ). (E) Schematic illustration representing the cell transplantation assay followed by Prox1 IF analysis. (F) Contribution of transplanted ECs to arterial and venous endothelial lineages. (G) Tg(fli1a:nEGFP)y7 donor cells derived from a wild-type (WT, top) or nova2uq14bh mutant (bottom) grafted into a WT acceptor embryo analyzed by Prox1 IF. Arrowheads indicate Prox1 and GFP double-positive mutant nuclei in the PCV. Arrow indicates Prox1-positive corpuscle of Stannius (CS). (H) Quantification of the percentage of Prox1 and GFP double-positive cells located in the dorsal side of PCV at 32 hpf (number of cells shown from sibling grafts (n = 21 grafts) and mutant grafts (n = 13 grafts) into wild-type hosts). Scale bars: 100 μm in (A), 500 μm in (B), 50 μm in inset figure, 20 μm in (C) and (Cʹ), 25 μm in (G). Error bars: SD, Mann-Whitney-test, ∗∗∗∗p < 0.0001, ns: not significant. See also Figure S3. |
|
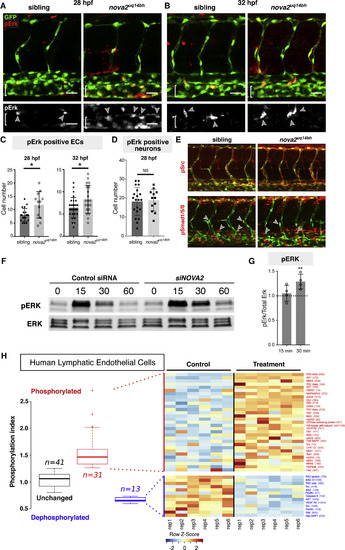
Nova2 Regulates MAPK-ERK Signaling (A and B) Phospho-Erk1/2 (pErk) immunostaining at 28 hpf (A) and 32 hpf (B) in Tg(fli1a:nEGFP)y7 siblings and nova2uq14bh mutants. Arrowheads indicate pErk-positive ECs (brackets indicate PCV). (C) Quantification of the number of pErk-positive ECs in the dorsal side of the PCV across 7 body segments at 28 hpf (siblings n = 20 and mutants n = 12) and 32 hpf (siblings n = 29 and mutants n = 20). (D) Quantification of the number of pErk-positive neurons across 3 body segments at 28 hpf. (siblings n = 20 and mutants n = 12). (E) Representative immunostains (32 hpf) for pSrc (upper, no qualitative differences observed) and for pSmad1/5/8 (lower, in which pSmad-positive arterial ECs and venous ECs were scored in the DA and PCV). No difference in the number of pSmad-positive cells across 4 somites was seen in DA (sib: 3.6 ± 1.6 [SD, n = 9], mut:4 ± 1.3 [n = 10]) or PCV (sib: 1.8 ± 1.5 [n = 9], mut:1.4 ± 1.3 [n = 10]). (F) Western blot analysis of ERK activation upon NOVA2knockdown in HuLECs stimulated with VEGFC. Representative of four independent blots, lower bands non-specific in both blots. (G) Quantitative analysis of normalized pERK expression (pErk/total Erk) relative to control siRNA levels from (F) shows prolonged activation of ERK at 30 min following VEGFC treatment. (H) Summary of phosphoarray analysis from human LECs. Left: Summary of overall experimental outcome with n = 41 unchanged phospho-sites, n = 31 increased phosphorylation events and n = 13 dephosphorylationevents. Right: Representative heat map of phosphoarray outcome. Increased and decreased phosphorylation events and targets are indicated. Data generated from n = 2 independent arrays with 6 independent assays for each Ab on each array. Error bars: SD, Scale bars: 25 μm, t test (D and G) or Mann-Whitney-test (C), ∗p < 0.05, ∗∗p < 0.01, and ns, not significant. See also Figure S5. |
Reprinted from Developmental Cell, 49, Baek, S., Oh, T.G., Secker, G., Sutton, D.L., Okuda, K.S., Paterson, S., Bower, N.I., Toubia, J., Koltowska, K., Capon, S.J., Baillie, G.J., Simons, C., Muscat, G.E.O., Lagendijk, A.K., Smith, K.A., Harvey, N.L., Hogan, B.M., The Alternative Splicing Regulator Nova2 Constrains Vascular Erk Signaling to Limit Specification of the Lymphatic Lineage, 279-292.e5, Copyright (2019) with permission from Elsevier. Full text @ Dev. Cell