- Title
-
Aggregation, segregation and dispersal of homotypic germ plasm RNPs in the early zebrafish embryo
- Authors
- Eno, C., Hansen, C.L., Pelegri, F.
- Source
- Full text @ Dev. Dyn.
|
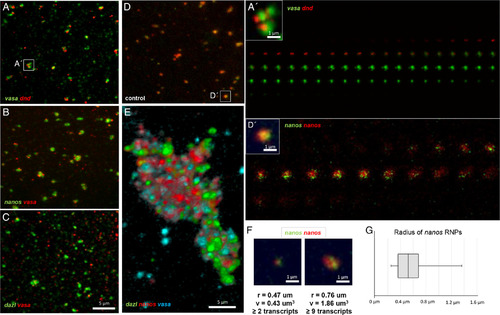
Zebrafish germline‐specific RNPs are homotypic and remain distinct during aggregation. Germ plasm RNAs as cortical pre‐aggregates (A–D) and furrow aggregate (E). Despite RNP‐RNP association, RNPs remain distinct and are homotypic, containing a single type of RNA. A–D:Pre‐aggregates at the cortex. Two different RNAs associate but do not fully colocalize (Pearson's coefficient: (A) vasa/dnd: r = 0.37, (B) nanos/vasa: r = 0.39, (C) dazl/vasa: r = 0.17, compared with a control experiment where the same RNA is targeted by identical probes that are labeled with two different fluorophores (D) nanos/nanos: r = |
|
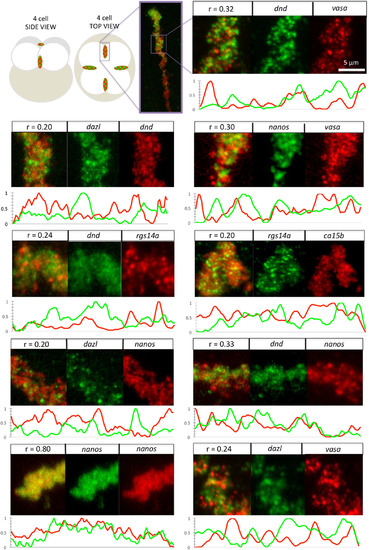
Correlation as a measure of RNA localization within germ plasm aggregates. Diagram of whole embryo with insets indicating location of furrow aggregates, followed by micrographs (merge and channel separated) of magnified regions. Furrow aggregates labeled to detect two germ plasm RNAs as indicated. Pearson's coefficient for combinations of two different RNAs ranges from 0.20 to 0.33, in contrast to control labelings for the same RNA targeted by differently labeled probes (nanos/nanos: r = 0.80). For each double labeling combination, 10‐μm section line scans of fluorescence intensity of separate channels along each aggregate show that fluorescence in embryos labeled for two different RNAs exhibit divergent peak distribution, in contrast with the control labeled nanos/nanosaggregate, where fluorescence distributions run largely in parallel. Fluorescence intensity values (y‐axis) are min‐max normalized to a scale of 0–1. All images are confocal Z‐projections. Scale bar = 5 μm. |
|
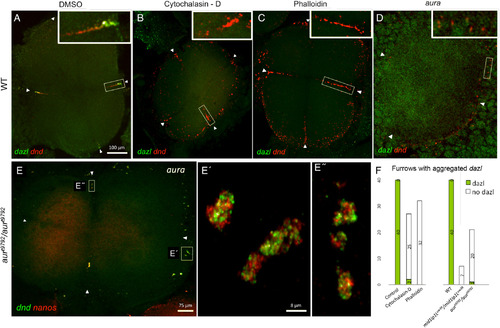
originally localized to the vegetal cortex, accumulates at the furrows (arrowheads) of the four cell‐staged blastocyst at the animal pole in wild‐type control (DMSO‐treated) embryos (A) but not in wild‐type embryos treated with F‐actin drugs cytochalasin‐D (B) and phalloidin (C), yet drug‐treated embryos exhibit germ plasm aggregate formation. D–E″:aura/mid1ip1Lmutant embryos show severely reduced dazl RNP accumulation at the furrow (Welch and Pelegri, 2015) (D), yet contain animal RNP aggregates (dnd/nanos, E, E″). F: Quantitation dnd RNP aggregates in the presence or absence of dazlRNPs: Panels A–E = confocal Z‐projections; Panels E′–E″ = SIM Z‐projections. Scale bars = 100 μm in A–D; 75 μm in E; 8 μm in E′–E″. |
|
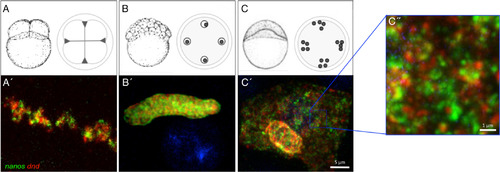
Germ plasm RNPs remain homotypic during asymmetric germ plasm segregation and dispersal. A–C″: The single‐type character of germ plasm RNPs appears to be maintained through aggregate formation at the furrows for the first several cell cycles (0–2 hpf [A,A′]), segregation in the blastula (2–4 hpf [B,B′]), and RNP dispersal at the end of the blastula period [4–6 hpf (C–C″]). Note in panels (C′,C″) RNPs are dispersed, largely occupying the cytoplasmic space, in contrast to aggregated, subcellularly localized germ plasm at earlier stages (A,B; see also Fig. 2 top left inset), Panel A′ = confocal Z‐projection; Panels B′–C″ = SIM Z‐projections. Scale bars = 5 μm in A′–C′; 1 μm in C″. Panels (A–C) adapted from Kimmel et al. (1995), with permission. |
|
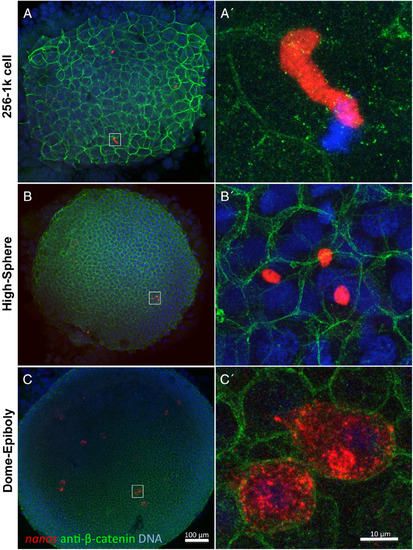
Germ plasm aggregates can undergo fragmentation before RNP dispersal. A,A′: In the early blastula embryo, elongated germ plasm aggregates appear intact and are present in a single blastomere. B,B′: In the mid‐blastula embryo, these aggregates can be observed to undergo fragmentation and are now present in multiple cells. C,C′: In the late blastula/early gastrula embryo, germ plasm aggregates undergo RNP cytoplasmic dispersal. Through the blastula stages (A–B′), germ plasm aggregates exhibit a distinct boundary with the cytoplasm, suggesting subcellular compartmentalization and indicating that germ plasm fragmentation occurs before germ plasm RNP dispersal. Germ plasm labeled using in situ hybridization to detect nanos RNA, cell membranes labeled with anti‐ß‐catenin antibodies, DNA labeled with DAPI. All images are confocal Z‐projections. Scale bars = 100 μm in A–C, 10 μm in A′–C′. |
|
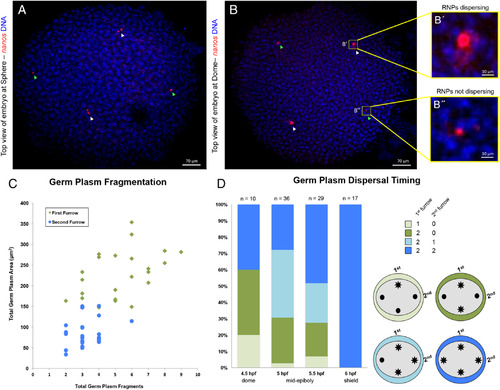
Germ plasm aggregate dispersal correlates with timing of RNP furrow recruitment. A,B: Top view of embryos demonstrating asynchrony in fragmentation and dispersal timing; germ plasm labeled with in situ hybridization for nanos and DNA with DAPI. A:Embryo at sphere stage with germ plasm aggregates localized to regions correlating to furrow origin. Germ plasm aggregates with inferred first furrow origin underwent fragmentation whereas second furrow aggregates did not. B: Embryo at dome stage with germ plasm aggregates correlating to furrow origin. In A,B, aggregates inferred to be derived from first and second furrow are labeled with white and green arrowheads, respectively. Germ plasm aggregate with inferred first furrow origin (B′) is undergoing RNP dispersal, while an aggregate with inferred second furrow origin (B″) is not. C:Correlation between germ plasm inferred furrow origin and aggregate fragmentation, n = 24 embryos. Total germ plasm area correlates (r = 0.66) with the number of germ plasm fragments in the same embryonic quadrant, with larger masses, inferred to have formed in the furrow for the first cell cycle, undergoing more fragmentation. D:Increase in frequency of RNP cytoplasmic dispersal through development and correlation between germ plasm inferred furrow origin and RNP dispersal. Embryos show patterns of germ plasm RNP cytoplasmic dispersal consistent with larger aggregates formed during first cell cycle furrow undergoing dispersal earlier than those formed during the second cell cycle. Other possible patterns of germ plasm RNP dispersal, such as a second furrow aggregate dispersing before either first furrow aggregate, were not observed. See text for details. All images are confocal Z‐projections. Scale bars = 70 μm A & B, 10 μm B′ & B″. |