- Title
-
Zebrafish Müller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons
- Authors
- Powell, C., Cornblath, E., Elsaeidi, F., Wan, J., Goldman, D.
- Source
- Full text @ Sci. Rep.
|
Injury models generate unique cell death signatures. (A) Representative images of retinal sections showing TUNEL detection (red) of apoptotic neuronal cell death 1 day following needle poke, UV light photoablation (PA), and NMDA neurotoxic injuries. (B) Relative localization of TUNEL+ nuclei by nuclear layer in the various injury models at 1 dpi. (C) Representative images of retinal sections showing TUNEL detection of apoptotic neuronal cell death at 1, 4 and 7 days following needle poke (single injury/retina), UV light photoablation, and NMDA neurotoxic injuries. (D) Quantification of TUNEL+ cells by nuclear layer in the various injury models at 1, 4 and 7 dpi. Data represents means ± s.d. (n = 4). Scale bar is equal to 50 μm. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; PA, photoablation; dpi, days post injury. |
|
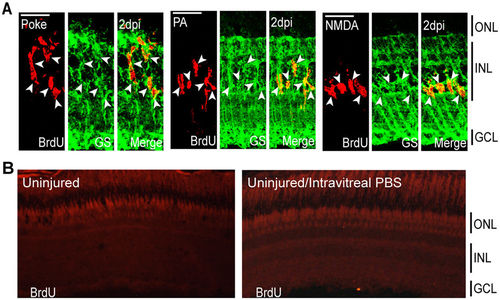
Injury models stimulate MG proliferation by 2 dpi in the INL. (A) Representative confocal images of retinal sections immunostained for BrdU and GS at 2 dpi following needle poke, PA, and NMDA injuries. Fish were given an injection of BrdU intraperitoneally 3 hours before harvest. Scale bar is equal to 50 μm. (B) PBS was injected into the vitreous of eyes whose retinas were uninjured. Fish were then given an injection of BrdU intraperitoneally 3 hours before harvest at 2 days post PBS injection. Shown are representative images of BrdU immunofluorescence in retinal sections. Similar results were obtained when BrdU was injected at 4 days post PBS injection. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; PA, photoablation; GS, glutamine synthetase; dpi, days post injury. |
|
Injury paradigms stimulate regenerative response signatures that exhibit both commonality and uniqueness. Fish were given an intraperitoneal injection of BrdU at 2 dpi and then sacrificed at 2 dpi (3 hours post BrdU injection), 4, 7, 14 and 30 dpi. (A–C) BrdU+ nuclei were counted and the percentage of BrdU+ nuclei residing in the (A) ONL, (B) INL and (C) GCL was determined for individual samples for each injury model. Data represents means ± s.d. (n ≥ 3). *P < 0.04549. (D–I) Representative images of retinal sections analyzed in (A–C) that were immunostained for BrdU at (D–F) 2 dpi or (G–I) 30 dpi following (D,G) needle poke, (E,H) PA or (F–I) NMDA injury. Scale bar is equal to 50 μm. Abbreviations are as in Fig. 1. |
|
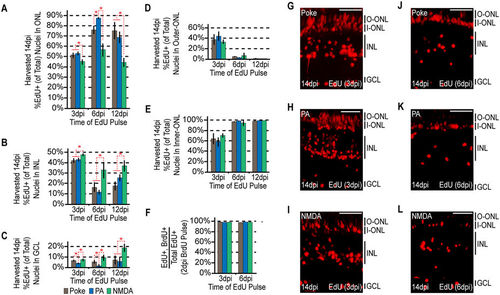
The ultimate localization of MG-derived progenitors becomes progressively more biased at later rounds of proliferation. Fish were given an intraperitoneal injection of BrdU at 2 dpi, followed by an injection of EdU at 3, 6, or 12 dpi. Each sample was harvested at 14dpi. (A–C) EdU+ nuclei were counted at the times indicated and the percentage of EdU+ nuclei residing in the (A) ONL, (B) INL and (C) GCL was determined for each injury model. Data represents means ± s.d. (n ≥ 3). *P < 0.02941. (D,E) The percentage of ONL EdU+ nuclei residing in (D) the upper region or (E) the lower region was determined for each injury model. (F) Co-staining samples for BrdU and EdU demonstrates that the cells proliferating at later times are a subpopulation of those proliferating at earlier times. (G–L) Representative images of retinal sections analyzed in (A–F) that were stained for EdU at (G–I) 3 dpi or (J–L) 6 dpi following (G,J) needle poke, (H,K) PA or (I–L) NMDA injury. Scale bar is equal to 50 μm. Abbreviations are as in Fig. 1. |
|
The localization of proliferating MG-derived progenitors by 6 dpi demonstrate injury-specific biases. Fish were given an injection of EdU 2 hours before harvesting at 6 dpi. (A) EdU+ cells were counted and the percentage of EdU+ nuclei residing in the ONL, INL, and GCL was determined for each injury model. Data represents means ± s.d. (n ≥ 3). *P < 0.02764. (B–D) Representative images of retinal sections analyzed in (A) that were stained for EdU following (B) needle poke, (C) PA or (D) NMDA injury. Scale bar is equal to 50 μm. Abbreviations are as in Fig. 1. |
|
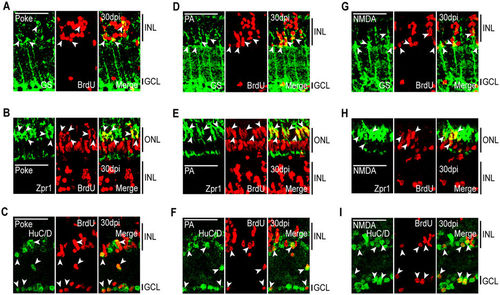
MG-derived progenitors are multipotent and generate unneeded neurons. Fish were given an intraperitoneal injection of BrdU at 2 dpi and sacrificed at 30 dpi. Immunostaining for BrdU and retinal cell type-specific markers was then performed against: GS, MG; Zpr1, red/green cones; HuC/D, amacrine and ganglion cells. Representative immunofluorescence images of retinal sections following (A–C) needle poke, (D–F) PA, or (G–I) NMDA injury. Retinal sections were stained with antibodies detecting BrdU and (A,D,G) GS, (B,E,H) Zpr1, or (C,F,I) HuC/D. Scale bar is equal to 50 μm. Abbreviations are as in Fig. 2. |