- Title
-
Zebrafish models of cerebrovascular disease
- Authors
- Walcott, B.P., and Peterson, R.T.
- Source
- Full text @ J. Cereb. Blood Flow Metab.
|
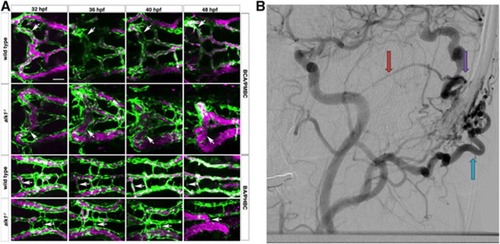
Phenotype comparison of zebrafish and human arteriovenous malformations (AVM). ( |
|
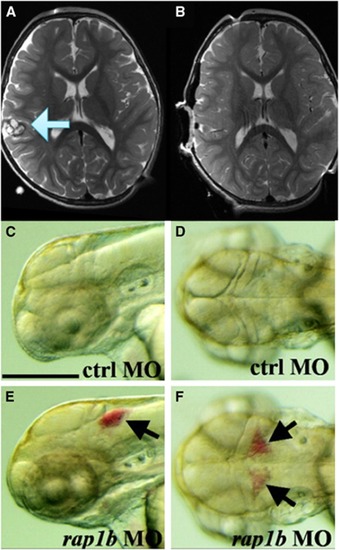
Phenotype comparison of zebrafish and cerebral cavernous malformation. In an magnetic resonance imaging of a human ( |